Nicotine promotes tumor growth and metastasis in mouse models of lung cancer
- PMID: 19841737
- PMCID: PMC2759510
- DOI: 10.1371/journal.pone.0007524
Nicotine promotes tumor growth and metastasis in mouse models of lung cancer
Erratum in
-
Correction: Nicotine Promotes Tumor Growth and Metastasis in Mouse Models of Lung Cancer.PLoS One. 2023 Dec 22;18(12):e0296477. doi: 10.1371/journal.pone.0296477. eCollection 2023. PLoS One. 2023. PMID: 38134017 Free PMC article.
Abstract
Background: Nicotine is the major addictive component of tobacco smoke. Although nicotine is generally thought to have limited ability to initiate cancer, it can induce cell proliferation and angiogenesis in a variety of systems. These properties might enable nicotine to facilitate the growth of tumors already initiated. Here we show that nicotine significantly promotes the progression and metastasis of tumors in mouse models of lung cancer. This effect was observed when nicotine was administered through intraperitoneal injections, or through over-the-counter transdermal patches.
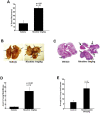
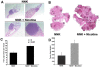
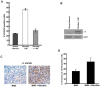
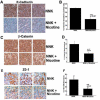
Methods and findings: In the present study, Line1 mouse adenocarcinoma cells were implanted subcutaneously into syngenic BALB/c mice. Nicotine administration either by intraperitoneal (i.p.) injection or transdermal patches caused a remarkable increase in the size of implanted Line1 tumors. Once the tumors were surgically removed, nicotine treated mice had a markedly higher tumor recurrence (59.7%) as compared to the vehicle treated mice (19.5%). Nicotine also increased metastasis of dorsally implanted Line1 tumors to the lungs by 9 folds. These studies on transplanted tumors were extended to a mouse model where the tumors were induced by the tobacco carcinogen, NNK. Lung tumors were initiated in A/J mice by i.p. injection of NNK; administration of 1 mg/kg nicotine three times a week led to an increase in the size and the number of tumors formed in the lungs. In addition, nicotine significantly reduced the expression of epithelial markers, E-Cadherin and beta-Catenin as well as the tight junction protein ZO-1; these tumors also showed an increased expression of the alpha(7) nAChR subunit. We believe that exposure to nicotine either by tobacco smoke or nicotine supplements might facilitate increased tumor growth and metastasis.
Conclusions: Our earlier results indicated that nicotine could induce invasion and epithelial-mesenchymal transition (EMT) in cultured lung, breast and pancreatic cancer cells. This study demonstrates for the first time that administration of nicotine either by i.p. injection or through over-the-counter dermal patches can promote tumor growth and metastasis in immunocompetent mice. These results suggest that while nicotine has only limited capacity to initiate tumor formation, it can facilitate the progression and metastasis of tumors pre-initiated by tobacco carcinogens.
Conflict of interest statement
Figures

References
-
- Brunnemann KD, Hoffmann D. Analytical studies on tobacco-specific N-nitrosamines in tobacco and tobacco smoke. Crit Rev Toxicol. 1991;21:235–240. - PubMed
-
- Hecht SS, Abbaspour A, Hoffman D. A study of tobacco carcinogenesis. XLII. Bioassay in A/J mice of some structural analogues of tobacco-specific nitrosamines. Cancer Lett. 1988;42:141–145. - PubMed
-
- Hecht SS. Cigarette smoking and lung cancer: chemical mechanisms and approaches to prevention. Lancet Oncol. 2002;3:461–469. - PubMed
-
- Sekido Y, Fong KM, Minna JD. Molecular genetics of lung cancer. Annu Rev Med. 2003;54:73–87. - PubMed
-
- Schuller HM, McGavin MD, Orloff M, Riechert A, Porter B. Simultaneous exposure to nicotine and hyperoxia causes tumors in hamsters. Lab Invest. 1995;73:448–456. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

