Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model
- PMID: 19842108
- PMCID: PMC2830796
- DOI: 10.1002/term.220
Donor-matched comparison of dental pulp stem cells and bone marrow-derived mesenchymal stem cells in a rat model
Abstract
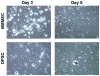
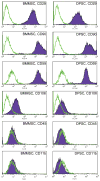
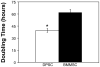
Dental pulp stem cells (DPSCs) have drawn much interest for the regeneration of mineralized tissues, and several studies have compared DPSCs to bone marrow-derived mesenchymal stem cells (BMMSCs). However, conflicting results, possibly due to donor-associated variability, have been published and the regenerative potential of DPSCs is currently unclear. In the present study we have sought to address this problem using a donor-matched experimental design to robustly compare the biological properties of DPSCs and BMMSCs. All experiments were performed using cells isolated from a single adult Sprague-Dawley rat. Our results show that DPSCs and BMMSCs had similar morphologies and flow cytometry profiles, were capable of forming colonies in vitro and were capable of osteogenic, chondrogenic and adipogenic differentiation. However, quantitative comparisons revealed that DPSCs had a faster population doubling time and a higher percentage of stem/progenitor cells in the population, as determined by clonogenic assays. Furthermore, while both cell populations formed mineral in vitro, DPSCs had significantly higher alkaline phosphatase activity than BMMSCs after 3 weeks in osteogenic medium. These data show several key differences between DPSCs and BMMSCs and support the possibility of using DPSCs for mineralized tissue regeneration.
2009 John Wiley & Sons, Ltd.
Conflict of interest statement
EJW is President and CEO of General BioTechnology, LLC. WSG is a medical director of General BioTechnology, LLC. Other authors report no potential conflicts of interest or financial interests.
Figures



Similar articles
-
A feasibility of useful cell-based therapy by bone regeneration with deciduous tooth stem cells, dental pulp stem cells, or bone-marrow-derived mesenchymal stem cells for clinical study using tissue engineering technology.Tissue Eng Part A. 2010 Jun;16(6):1891-900. doi: 10.1089/ten.TEA.2009.0732. Tissue Eng Part A. 2010. PMID: 20067397
-
Comparison of immunomodulatory properties of exosomes derived from bone marrow mesenchymal stem cells and dental pulp stem cells.Immunol Res. 2019 Oct;67(4-5):432-442. doi: 10.1007/s12026-019-09088-6. Immunol Res. 2019. PMID: 31407157
-
Diagnostic Cytokines and Comparative Analysis Secreted from Exfoliated Deciduous Teeth, Dental Pulp, and Bone Marrow Derived Mesenchymal Stem Cells for Functional Cell-Based Therapy.Int J Mol Sci. 2019 Nov 24;20(23):5900. doi: 10.3390/ijms20235900. Int J Mol Sci. 2019. PMID: 31771293 Free PMC article.
-
Mesenchymal stem cells derived from dental tissues vs. those from other sources: their biology and role in regenerative medicine.J Dent Res. 2009 Sep;88(9):792-806. doi: 10.1177/0022034509340867. J Dent Res. 2009. PMID: 19767575 Free PMC article. Review.
-
Comparative Dental Pulp Stem Cells (DPSCs) and Periodontal Ligament Stem Cells (PDLSCs): Difference in effect of aspirin on osteoblast potential of PDLSCs and DPSCs.Tissue Cell. 2025 Jun;94:102776. doi: 10.1016/j.tice.2025.102776. Epub 2025 Feb 21. Tissue Cell. 2025. PMID: 40022908 Review.
Cited by
-
In-vitro analysis on the potential use of dental pulp mesenchymal stem cells on arecoline-induced oral epithelial cells.Med Oncol. 2022 Feb 23;39(5):77. doi: 10.1007/s12032-022-01673-4. Med Oncol. 2022. PMID: 35195802
-
Amphiregulin regulates odontogenic differentiation of dental pulp stem cells by activation of mitogen-activated protein kinase and the phosphatidylinositol 3-kinase signaling pathways.Stem Cell Res Ther. 2022 Jul 15;13(1):304. doi: 10.1186/s13287-022-02971-4. Stem Cell Res Ther. 2022. PMID: 35841013 Free PMC article.
-
Transplantation of dental pulp stem cells suppressed inflammation in sciatic nerves by promoting macrophage polarization towards anti-inflammation phenotypes and ameliorated diabetic polyneuropathy.J Diabetes Investig. 2016 Jul;7(4):485-96. doi: 10.1111/jdi.12452. Epub 2015 Dec 31. J Diabetes Investig. 2016. PMID: 27181261 Free PMC article.
-
Cell sheets of human dental pulp stem cells for future application in bone replacement.Clin Oral Investig. 2019 Jun;23(6):2713-2721. doi: 10.1007/s00784-018-2630-8. Epub 2018 Oct 24. Clin Oral Investig. 2019. PMID: 30357480
-
Dental Pulp Stem Cells: An Attractive Alternative for Cell Therapy in Ischemic Stroke.Front Neurol. 2019 Aug 2;10:824. doi: 10.3389/fneur.2019.00824. eCollection 2019. Front Neurol. 2019. PMID: 31428038 Free PMC article. Review.
References
-
- Castro-Malaspina H, Gay RE, Resnick G, Kapoor N, Meyers P, Chiarieri D, McKenzie S, Broxmeyer HE, Moore MA. Characterization of human bone marrow fibroblast colony-forming cells (CFU-F) and their progeny. Blood. 1980;56(2):289–301. - PubMed
-
- d’Aquino R, Graziano A, Sampaolesi M, Laino G, Pirozzi G, De Rosa A, Papaccio G. Human postnatal dental pulp cells co-differentiate into osteoblasts and endotheliocytes: a pivotal synergy leading to adult bone tissue formation. Cell Death Differ. 2007;14(6):1162–71. - PubMed
-
- Derubeis AR, Cancedda R. Bone marrow stromal cells (BMSCs) in bone engineering: limitations and recent advances. Ann Biomed Eng. 2004;32(1):160–5. - PubMed
-
- Gay IC, Chen S, MacDougall M. Isolation and characterization of multipotent human periodontal ligament stem cells. Orthod Craniofac Res. 2007;10(3):149–60. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

