Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry
- PMID: 19843011
- PMCID: PMC2860680
- DOI: 10.1042/BJ20090884
Role of phosphoinositides in STIM1 dynamics and store-operated calcium entry
Abstract
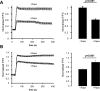
Ca2+ entry through store-operated Ca2+ channels involves the interaction at ER-PM (endoplasmic reticulum-plasma membrane) junctions of STIM (stromal interaction molecule) and Orai. STIM proteins are sensors of the luminal ER Ca2+ concentration and, following depletion of ER Ca2+, they oligomerize and translocate to ER-PM junctions where they form STIM puncta. Direct binding to Orai proteins activates their Ca2+ channel function. It has been suggested that an additional interaction of the C-terminal polybasic domain of STIM1 with PM phosphoinositides could contribute to STIM1 puncta formation prior to binding to Orai. In the present study, we investigated the role of phosphoinositides in the formation of STIM1 puncta and SOCE (store-operated Ca2+ entry) in response to store depletion. Treatment of HeLa cells with inhibitors of PI3K (phosphatidylinositol 3-kinase) and PI4K (phosphatidylinositol 4-kinase) (wortmannin and LY294002) partially inhibited formation of STIM1 puncta. Additional rapid depletion of PtdIns(4,5)P2 resulted in more substantial inhibition of the translocation of STIM1-EYFP (enhanced yellow fluorescent protein) into puncta. The inhibition was extensive at a concentration of LY294002 (50 microM) that should primarily inhibit PI3K, consistent with a major role for PtdIns(4,5)P2 and PtdIns(3,4,5)P3 in puncta formation. Depletion of phosphoinositides also inhibited SOCE based on measurement of the rise in intracellular Ca2+ concentration after store depletion. Overexpression of Orai1 resulted in a recovery of translocation of STMI1 into puncta following phosphoinositide depletion and, under these conditions, SOCE was increased to above control levels. These observations support the idea that phosphoinositides are not essential but contribute to STIM1 accumulation at ER-PM junctions with a second translocation mechanism involving direct STIM1-Orai interactions.
Figures





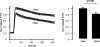
References
-
- Parekh A. B., Putney J. W., Jr Store-operated calcium channels. Physiol. Rev. 2005;85:757–810. - PubMed
-
- Putney J. W., Jr A model for receptor-regulated calcium entry. Cell Calcium. 1986;7:1–12. - PubMed
-
- Feske S., Gwack Y., Prakriya M., Srikanth S., Puppel S. H., Tanasa B., Hogan P. G., Lewis R. S., Daly M., Rao A. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441:179–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

