Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance
- PMID: 19843452
- PMCID: PMC2764069
- DOI: 10.1016/j.bpj.2009.08.003
Two-path impedance spectroscopy for measuring paracellular and transcellular epithelial resistance
Abstract
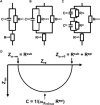
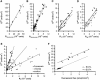
Solutes are transported across epithelial cell layers via transcellular and paracellular pathways. The transcellular pathway leads across the apical and basolateral cell membrane, whereas the paracellular pathway is directed through the tight junction. Tight junction proteins (claudins, occludin, tricellulin) can not only form barriers but also paracellular channels that are--in concert with membrane channels and transporters--regulated in a wide range in health and disease states. Thus, it is desirable to determine para- and transcellular resistance (R(para), R(trans)) separately. This cannot be achieved by conventional transepithelial resistance (TER) measurements. We present an impedance spectroscopic approach that is optimized for differentiation between these two pathways. The method is based on a transepithelial impedance measurement in specialized Ussing chambers, combined with a Ca(2+)-dependent modulation of R(para) through EGTA and flux measurements of a nonradioactive paracellular marker, fluorescein. The prerequisites are a paracellular marker that varies in parallel to 1/R(para), an experimental regime that alters R(para) without affecting R(trans), and exact knowledge of the resistance of subepithelial components. The underlying prerequisites and the applicability as a routine method were verified on cell lines of different tightness including HT-29/B6 colon cells and Madin-Darby canine kidney tubule cells C7 and C11.
Figures
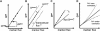



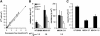
References
-
- Tsukita S., Furuse M., Itoh M. Multifunctional strands in tight junctions. Nat. Rev. Mol. Cell Biol. 2001;2:285–293. - PubMed
-
- Amasheh S., Meiri N., Gitter A.H., Schöneberg T., Mankertz J. Claudin-2 expression induces cation-selective channels in tight junctions of epithelial cells. J. Cell Sci. 2002;115:4969–4976. - PubMed
-
- Günzel D., Stuiver M., Kausalya P.J., Haisch L., Krug S.M. Claudin-10 exists in six alternatively spliced isoforms which exhibit distinct localization and function. J. Cell Sci. 2009;122:1507–1517. - PubMed
-
- Mankertz J., Schulzke J.D. Altered permeability in inflammatory bowel disease: pathophysiology and clinical implications. Curr. Opin. Gastroenterol. 2007;23:379–383. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

