Spatial and temporal dynamics of mitochondrial membrane permeability waves during apoptosis
- PMID: 19843454
- PMCID: PMC2764100
- DOI: 10.1016/j.bpj.2009.07.056
Spatial and temporal dynamics of mitochondrial membrane permeability waves during apoptosis
Abstract
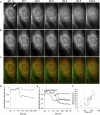
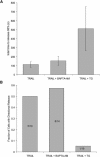
Change in the permeability of the mitochondrial membrane to proteins (cytochrome c and Smac) and protons is a critical step in apoptosis. Although the time from the induction of apoptosis to the change of mitochondrial permeability is variable over a period of hours, the release of proteins is an "all or none" phenomenon that is completed in an individual cell within minutes. Here, using single-cell fluorescence microscopy, we show that the release of cytochrome c from a single mitochondrion occurs in a single step. However, this increased permeability of the outer membrane to cytochrome c propagates throughout the cell as a slower, spatially coordinated wave. The permeability of the outer membrane to Smac propagates with the same spatial pattern but lagging in time. This is followed by a wave of increased permeability of the inner membrane to protons. Only afterward do the mitochondria fission. The spatial dependence of the permeability wave was inhibited by thapsigargin, an inhibitor of the endoplasmic reticulum calcium pumps, but buffering cytosolic calcium had no effect. These results show that the trigger for apoptosis is spatially localized, initiating at one or only a few mitochondria preceding the loss of mitochondrial energetics, and the subsequent temporal propagation of mitochondrial membrane permeability is calcium-dependent.
Figures



References
-
- Ellis R.E., Yuan J.Y., Horvitz H.R. Mechanisms and functions of cell death. Annu. Rev. Cell Biol. 1991;7:663–698. - PubMed
-
- Wyllie A.H. Glucocorticoid-induced thymocyte apoptosis is associated with endogenous endonuclease activation. Nature. 1980;284:555–556. - PubMed
-
- Yuan J., Shaham S., Ledoux S., Ellis H.M., Horvitz H.R. The C. elegans cell death gene ced-3 encodes a protein similar to mammalian interleukin-1 β-converting enzyme. Cell. 1993;75:641–652. - PubMed
-
- Youle R.J., Strasser A. The BCL-2 protein family: opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008;9:47–59. - PubMed
-
- Frezza C., Cipolat S., Martins de Brito O., Micaroni M., Beznoussenko G.V. OPA1 controls apoptotic cristae remodeling independently from mitochondrial fusion. Cell. 2006;126:177–189. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

