IRS1 regulation by Wnt/beta-catenin signaling and varied contribution of IRS1 to the neoplastic phenotype
- PMID: 19843521
- PMCID: PMC2804351
- DOI: 10.1074/jbc.M109.060319
IRS1 regulation by Wnt/beta-catenin signaling and varied contribution of IRS1 to the neoplastic phenotype
Abstract
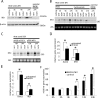
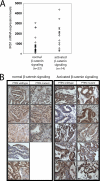
Dysregulation of beta-catenin levels and localization and constitutive activation of beta-catenin/TCF (T cell factor)-regulated gene expression occur in many cancers, including the majority of colorectal carcinomas and a subset of ovarian endometrioid adenocarcinomas. Based on the results of microarray-based gene expression profiling we found the insulin receptor substrate 1 (IRS1) gene as one of the most highly up-regulated genes upon ectopic expression of a mutant, constitutively active form of beta-catenin in the rat kidney epithelial cell line RK3E. We demonstrate expression of IRS1 can be directly activated by beta-catenin, likely in part via beta-catenin/TCF binding to TCF consensus binding elements located in the first intron and downstream of the IRS1 transcriptional start site. Consistent with the proposal that beta-catenin is an important regulator of IRS1 expression in vivo, we observed that IRS1 is highly expressed in many cancers with constitutive stabilization of beta-catenin, such as colorectal carcinomas and ovarian endometrioid adenocarcinomas. Using a short hairpin RNA approach to abrogate IRS1 expression and function, we found that IRS1 function is required for efficient de novo neoplastic transformation by beta-catenin in RK3E cells. Our findings add to the growing body of data implicating IRS1 as a critical signaling component in cancer development and progression.
Figures


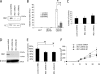
References
-
- Moon R. T., Kohn A. D., De Ferrari G. V., Kaykas A. (2004) Nat. Rev. Genet. 5, 691–701 - PubMed
-
- Clevers H. (2006) Cell 127, 469–480 - PubMed
-
- Polakis P. (2007) Curr. Opin. Genet. Dev. 17, 45–51 - PubMed
-
- Städeli R., Hoffmans R., Basler K. (2006) Curr. Biol. 16, R378–R385 - PubMed
-
- Sakatani T., Kaneda A., Iacobuzio-Donahue C. A., Carter M. G., de Boom Witzel S., Okano H., Ko M. S., Ohlsson R., Longo D. L., Feinberg A. P. (2005) Science 307, 1976–1978 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

