Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles
- PMID: 19843540
- PMCID: PMC2798719
- DOI: 10.1093/hmg/ddp483
Tsc/mTORC1 signaling in oocytes governs the quiescence and activation of primordial follicles
Abstract
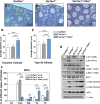
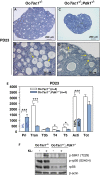
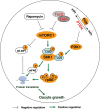
To maintain the female reproductive lifespan, the majority of ovarian primordial follicles are preserved in a quiescent state in order to provide ova for later reproductive life. However, the molecular mechanism that maintains the long quiescence of primordial follicles is poorly understood. Here we provide genetic evidence to show that the tumor suppressor tuberous sclerosis complex 1 (Tsc1), which negatively regulates mammalian target of rapamycin complex 1 (mTORC1), functions in oocytes to maintain the quiescence of primordial follicles. In mutant mice lacking the Tsc1 gene in oocytes, the entire pool of primordial follicles is activated prematurely due to elevated mTORC1 activity in the oocyte, ending up with follicular depletion in early adulthood and causing premature ovarian failure (POF). We further show that maintenance of the quiescence of primordial follicles requires synergistic, collaborative functioning of both Tsc and PTEN (phosphatase and tensin homolog deleted on chromosome 10) and that these two molecules suppress follicular activation through distinct ways. Our results suggest that Tsc/mTORC1 signaling and PTEN/PI3K (phosphatidylinositol 3 kinase) signaling synergistically regulate the dormancy and activation of primordial follicles, and together ensure the proper length of female reproductive life. Deregulation of these signaling pathways in oocytes results in pathological conditions of the ovary, including POF and infertility.
Figures
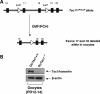
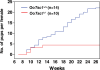
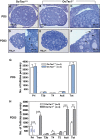
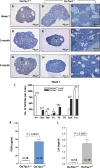


References
-
- Hirshfield A.N. Development of follicles in the mammalian ovary. Int. Rev. Cytol. 1991;124:43–101. - PubMed
-
- McGee E.A., Hsueh A.J. Initial and cyclic recruitment of ovarian follicles. Endocr. Rev. 2000;21:200–214. - PubMed
-
- Adhikari D., Liu K. Molecular mechanisms underlying the activation of mammalian primordial follicles. Endocr. Rev. 2009;30:438–464. - PubMed
-
- Broekmans F.J., Knauff E.A., te Velde E.R., Macklon N.S., Fauser B.C. Female reproductive ageing: current knowledge and future trends. Trends Endocrinol. Metab. 2007;18:58–65. - PubMed
-
- Faddy M.J., Gosden R.G., Gougeon A., Richardson S.J., Nelson J.F. Accelerated disappearance of ovarian follicles in mid-life: implications for forecasting menopause. Hum. Reprod. 1992;7:1342–1346. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

