An automated and multiplexed method for high throughput peptide immunoaffinity enrichment and multiple reaction monitoring mass spectrometry-based quantification of protein biomarkers
- PMID: 19843560
- PMCID: PMC2808264
- DOI: 10.1074/mcp.M900254-MCP200
An automated and multiplexed method for high throughput peptide immunoaffinity enrichment and multiple reaction monitoring mass spectrometry-based quantification of protein biomarkers
Abstract
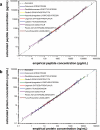
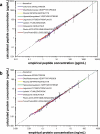
There is an urgent need for quantitative assays in verifying and validating the large numbers of protein biomarker candidates produced in modern "-omics" experiments. Stable isotope standards with capture by anti-peptide antibodies (SISCAPA) has shown tremendous potential to meet this need by combining peptide immunoaffinity enrichment with quantitative mass spectrometry. In this study, we describe three significant advances to the SISCAPA technique. First, we develop a method for an automated magnetic bead-based platform capable of high throughput processing. Second, we implement the automated method in a multiplexed SISCAPA assay (nine targets in one assay) and assess the performance characteristics of the multiplexed assay. Using the automated, multiplexed platform, we demonstrate detection limits in the physiologically relevant ng/ml range (from 10 microl of plasma) with sufficient precision (median coefficient of variation, 12.6%) for quantifying biomarkers. Third, we demonstrate that enrichment of peptides from larger volumes of plasma (1 ml) can extend the limits of detection to the low pg/ml range of protein concentration. The method is generally applicable to any protein or biological specimen of interest and holds great promise for analyzing large numbers of biomarker candidates.
Figures
References
-
- Preissner C. M., O'Kane D. J., Singh R. J., Morris J. C., Grebe S. K. (2003) Phantoms in the assay tube: Heterophile antibody interferences in serum thyroglobulin assays. J. Clin. Endocrinol. Metab. 88, 3069–3074 - PubMed
-
- Rifai N., Gillette M. A., Carr S. A. (2006) Protein biomarker discovery and validation: the long and uncertain path to clinical utility. Nat. Biotechnol. 24, 971–983 - PubMed
-
- Anderson N. L., Anderson N. G., Haines L. R., Hardie D. B., Olafson R. W., Pearson T. W. (2004) Mass spectrometric quantitation of peptides and proteins using stable isotope standards and capture by anti-peptide antibodies (SISCAPA). J. Proteome Res. 3, 235–244 - PubMed
-
- Whiteaker J. R., Zhang H., Zhao L., Wang P., Kelly-Spratt K. S., Ivey R. G., Piening B. D., Feng L. C., Kasarda E., Gurley K. E., Eng J. K., Chodosh L. A., Kemp C. J., McIntosh M. W., Paulovich A. G. (2007) Integrated pipeline for mass spectrometry-based discovery and confirmation of biomarkers demonstrated in a mouse model of breast cancer. J. Proteome Res. 6, 3962–3975 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

