Protein kinase A-dependent biophysical phenotype for V227F-KCNJ2 mutation in catecholaminergic polymorphic ventricular tachycardia
- PMID: 19843922
- PMCID: PMC2766080
- DOI: 10.1161/CIRCEP.109.872309
Protein kinase A-dependent biophysical phenotype for V227F-KCNJ2 mutation in catecholaminergic polymorphic ventricular tachycardia
Abstract
Background: KCNJ2 encodes Kir2.1, a pore-forming subunit of the cardiac inward rectifier current, I(K1). KCNJ2 mutations are associated with Andersen-Tawil syndrome and catecholaminergic polymorphic ventricular tachycardia. The aim of this study was to characterize the biophysical and cellular phenotype of a KCNJ2 missense mutation, V227F, found in a patient with catecholaminergic polymorphic ventricular tachycardia.
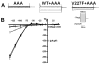
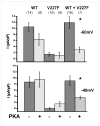
Methods and results: Kir2.1-wild-type (WT) and V227F channels were expressed individually and together in Cos-1 cells to measure I(K1) by voltage clamp. Unlike typical Andersen-Tawil syndrome-associated KCNJ2 mutations, which show dominant negative loss of function, Kir2.1WT+V227F coexpression yielded I(K1) indistinguishable from Kir2.1-WT under basal conditions. To simulate catecholamine activity, a protein kinase A (PKA)-stimulating cocktail composed of forskolin and 3-isobutyl-1-methylxanthine was used to increase PKA activity. This PKA-simulated catecholaminergic stimulation caused marked reduction of outward I(K1) compared with Kir2.1-WT. PKA-induced reduction in I(K1) was eliminated by mutating the phosphorylation site at serine 425 (S425N).
Conclusions: Heteromeric Kir2.1-V227F and WT channels showed an unusual latent loss of function biophysical phenotype that depended on PKA-dependent Kir2.1 phosphorylation. This biophysical phenotype, distinct from typical Andersen-Tawil syndrome mutations, suggests a specific mechanism for PKA-dependent I(K1) dysfunction for this KCNJ2 mutation, which correlates with adrenergic conditions underlying the clinical arrhythmia.
Figures




References
-
- Wible BA, De Biasi M, Majumder K, Taglialatela M, Brown AM. Cloning and functional expression of an inwardly rectifying K+ channel from human atrium. Circ Res. 1995;76:343–350. - PubMed
-
- Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen’s syndrome. Cell. 2001;105:511–519. - PubMed
-
- Priori SG, Pandit SV, Rivolta I, Berenfeld O, Ronchetti E, Dhamoon A, Napolitano C, Anumonwo J, di Barletta MR, Gudapakkam S, Bosi G, Stramba-Badiale M, Jalife J. A novel form of short QT syndrome (SQT3) is caused by a mutation in the KCNJ2 gene. Circ Res. 2005;96:800–807. - PubMed
-
- Xia M, Jin Q, Bendahhou S, He Y, Larroque MM, Chen Y, Zhou Q, Yang Y, Liu Y, Liu B, Zhu Q, Zhou Y, Lin J, Liang B, Li L, Dong X, Pan Z, Wang R, Wan H, Qiu W, Xu W, Eurlings P, Barhanin J, Chen Y. A Kir2.1 gain-of-function mutation underlies familial atrial fibrillation. Biochem Biophys Res Commun. 2005;332:1012–1019. - PubMed
-
- Donaldson MR, Yoon G, Fu YH, Ptacek LJ. Andersen-Tawil syndrome: a model of clinical variability, pleiotropy, and genetic heterogeneity. Ann Med. 2004;36 (Suppl 1):92–97. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

