1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3
- PMID: 19843932
- PMCID: PMC2810518
- DOI: 10.4049/jimmunol.0803217
1,25-Dihydroxyvitamin D3 and IL-2 combine to inhibit T cell production of inflammatory cytokines and promote development of regulatory T cells expressing CTLA-4 and FoxP3
Abstract
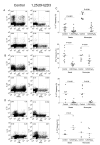
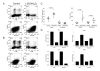
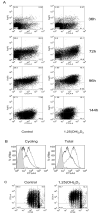
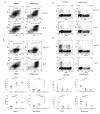
The active form of vitamin D, 1,25-dihydroxyvitamin D(3) (1,25(OH)(2)D(3)), has potent immunomodulatory properties that have promoted its potential use in the prevention and treatment of infectious disease and autoimmune conditions. A variety of immune cells, including macrophages, dendritic cells, and activated T cells express the intracellular vitamin D receptor and are responsive to 1,25(OH)(2)D(3.) Despite this, how 1,25(OH)(2)D(3) regulates adaptive immunity remains unclear and may involve both direct and indirect effects on the proliferation and function of T cells. To further clarify this issue, we have assessed the effects of 1,25(OH)(2)D(3) on human CD4(+)CD25(-) T cells. We observed that stimulation of CD4(+)CD25(-) T cells in the presence of 1,25(OH)(2)D(3) inhibited production of proinflammatory cytokines including IFN- gamma, IL-17, and IL-21 but did not substantially affect T cell division. In contrast to its inhibitory effects on inflammatory cytokines, 1,25(OH)(2)D(3) stimulated expression of high levels of CTLA-4 as well as FoxP3, the latter requiring the presence of IL-2. T cells treated with 1,25(OH)(2)D(3) could suppress proliferation of normally responsive T cells, indicating that they possessed characteristics of adaptive regulatory T cells. Our results suggest that 1,25(OH)(2)D(3) and IL-2 have direct synergistic effects on activated T cells, acting as potent anti-inflammatory agents and physiologic inducers of adaptive regulatory T cells.
Figures



References
-
- O’Garra A, Murphy K. Role of cytokines in determining T-lymphocyte function. Curr Opin Immunol. 1994;6:458–466. - PubMed
-
- Bettelli E, Oukka M, Kuchroo VK. T(H)-17 cells in the circle of immunity and autoimmunity. Nat Immunol. 2007;8:345–350. - PubMed
-
- Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

