A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity
- PMID: 19843940
- PMCID: PMC6292203
- DOI: 10.4049/jimmunol.0900447
A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity
Abstract
To generate chimeric Ag receptors (CARs) for the adoptive immunotherapy of cancer patients with ErbB2-expressing tumors, a single-chain Ab derived from the humanized mAb 4D5 Herceptin (trastuzumab) was initially linked to T cell signaling domains derived from CD28 and the CD3zeta to generate a CAR against ErbB2. Human PBLs expressing the 4D5 CAR demonstrated Ag-specific activities against ErbB2(+) tumors. However, a gradual loss of transgene expression was noted for PBLs transduced with this 4D5 CAR. When the CD3zeta signaling domain of the CAR was truncated or mutated, loss of CAR expression was not observed, suggesting that the CD3zeta signaling caused the transgene decrease, which was supported by the finding that T cells expressing 4D5 CARs with CD3zeta ITAM mutations were less prone to apoptosis. By adding 4-1BB cytoplasmic domains to the CD28-CD3zeta signaling moieties, we found increased transgene persistence in 4D5 CAR-transduced PBLs. Furthermore, constructs with 4-1BB sequences demonstrated increased cytokine secretion and lytic activity in 4D5 CAR-transduced T cells. More importantly, PBLs expressing this new version of the 4D5 CAR could not only efficiently lyse the autologous fresh tumor digests, but they could strongly suppress tumor growth in a xenogenic mouse model.
Conflict of interest statement
Disclosures
The authors have no financial conflicts of interest.
Figures





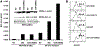
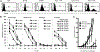
References
-
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL,Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, et al. 2005. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol 23: 2346–2357. - PMC - PubMed
-
- Sadelain M, Riviere I, and Brentjens R. 2003. Targeting tumours with genetically enhanced T lymphocytes. Nat. Rev. Cancer 3: 35–45. - PubMed
-
- Murphy A, Westwood JA, Teng MW, Moeller M, Darcy PK, and Kershaw MH. 2005. Gene modification strategies to induce tumor immunity. Immunity 22: 403–414. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

