Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice
- PMID: 19844159
- PMCID: PMC2873687
- DOI: 10.4161/auto.5.8.9991
Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice
Abstract
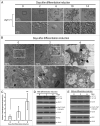
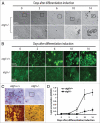
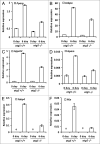
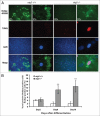
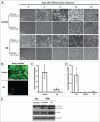
Mammalian white adipocytes have a unique structure in which nearly the entire cell volume is occupied by a single large lipid droplet, while the surrounding cytoplasm occupies minimal space. The massive cytoplasmic remodeling processes involved in the formation of this unique cellular structure are poorly defined. Autophagy is a membrane trafficking process leading to lysosomal degradation of cytoplasmic components. Here, we investigated the functional role of atg5, a gene encoding an essential protein required for autophagy, in adipocyte differentiation in a cellular model and in mice. Massive autophagy was activated when wild-type primary mouse embryonic fibroblasts (MEFs) were induced for adipocyte differentiation. Importantly, the autophagy deficient primary atg5(-/-) MEFs exhibited dramatically reduced efficiency in adipogenesis. Time-lapse microscopy revealed that atg5(-/-) MEFs initially appeared to differentiate normally; however, a majority of the differentiating atg5(-/-) cells ultimately failed to undergo further morphological transformation and eventually died, likely through apoptosis. Consistent with these in vitro results, histological analysis revealed that the atg5(-/-) late-stage embryos and neonatal pups had much less subcutaneous perilipin A-positive adipocytes. Consistently, when treated with chloroquine, a functional inhibitor of autophagy, wild-type MEFs exhibited drastically reduced efficiency of adipocyte differentiation. Taken together, these findings demonstrated that Atg5 is involved in normal adipocyte differentiation, suggesting an important role of autophagy in adipogenesis.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
