Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth
- PMID: 19844197
- PMCID: PMC2839205
- DOI: 10.1038/mt.2009.237
Adipose tissue-derived mesenchymal stem cells expressing prodrug-converting enzyme inhibit human prostate tumor growth
Abstract
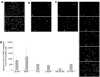
The ability of human adipose tissue-derived mesenchymal stem cells (AT-MSCs), engineered to express the suicide gene cytosine deaminase::uracil phosphoribosyltransferase (CD::UPRT), to convert the relatively nontoxic 5-fluorocytosine (5-FC) into the highly toxic antitumor 5-fluorouracil (5-FU) together with their ability to track and engraft into tumors and micrometastases makes these cells an attractive tool to activate prodrugs directly within the tumor mass. In this study, we tested the feasibility and efficacy of these therapeutic cells to function as cellular vehicles of prodrug-activating enzymes in prostate cancer (PC) therapy. In in vitro migration experiments we have shown that therapeutic AT-MSCs migrated to all the prostate cell lines tested. In a pilot preclinical study, we observed that coinjections of human bone metastatic PC cells along with the transduced AT-MSCs into nude mice treated with 5-FC induced a complete tumor regression in a dose dependent manner or did not even allow the establishment of the tumor. More importantly, we also demonstrated that the therapeutic cells were effective in significantly inhibiting PC tumor growth after intravenous administration that is a key requisite for any clinical application of gene-directed enzyme prodrug therapies.
Figures

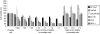


Similar articles
-
Genetically engineered theranostic mesenchymal stem cells for the evaluation of the anticancer efficacy of enzyme/prodrug systems.J Control Release. 2015 Feb 28;200:179-87. doi: 10.1016/j.jconrel.2015.01.003. Epub 2015 Jan 7. J Control Release. 2015. PMID: 25575867 Free PMC article.
-
Mesenchymal stem cells expressing therapeutic genes induce autochthonous prostate tumour regression.Eur J Cancer. 2014 Sep;50(14):2478-88. doi: 10.1016/j.ejca.2014.06.014. Epub 2014 Jul 21. Eur J Cancer. 2014. PMID: 25060826
-
Human adipose tissue-derived mesenchymal stem cells expressing yeast cytosinedeaminase::uracil phosphoribosyltransferase inhibit intracerebral rat glioblastoma.Int J Cancer. 2012 May 15;130(10):2455-63. doi: 10.1002/ijc.26278. Epub 2011 Aug 24. Int J Cancer. 2012. PMID: 21732344
-
Stem cell based gene therapy in prostate cancer.Biomed Res Int. 2014;2014:549136. doi: 10.1155/2014/549136. Epub 2014 Jul 10. Biomed Res Int. 2014. PMID: 25121103 Free PMC article. Review.
-
Microbial cytosine deaminase is a programmable anticancer prodrug mediating enzyme: antibody, and gene directed enzyme prodrug therapy.Heliyon. 2022 Sep 16;8(9):e10660. doi: 10.1016/j.heliyon.2022.e10660. eCollection 2022 Sep. Heliyon. 2022. PMID: 36164544 Free PMC article. Review.
Cited by
-
Tumor-Targeted Immunotherapy by Using Primary Adipose-Derived Stem Cells and an Antigen-Specific Protein Vaccine.Cancers (Basel). 2018 Nov 15;10(11):446. doi: 10.3390/cancers10110446. Cancers (Basel). 2018. PMID: 30445793 Free PMC article.
-
Mesenchymal stem cell-released oncolytic virus: an innovative strategy for cancer treatment.Cell Commun Signal. 2023 Feb 24;21(1):43. doi: 10.1186/s12964-022-01012-0. Cell Commun Signal. 2023. PMID: 36829187 Free PMC article. Review.
-
Targeting of CD133+ Cancer Stem Cells by Mesenchymal Stem Cell Expressing TRAIL Reveals a Prospective Role of Apoptotic Gene Regulation in Non-Small Cell Lung Cancer.Cancers (Basel). 2019 Aug 28;11(9):1261. doi: 10.3390/cancers11091261. Cancers (Basel). 2019. PMID: 31466290 Free PMC article.
-
Preclinical Study on Biodistribution of Mesenchymal Stem Cells after Local Transplantation into the Brain.Int J Stem Cells. 2023 Nov 30;16(4):415-424. doi: 10.15283/ijsc23062. Epub 2023 Aug 30. Int J Stem Cells. 2023. PMID: 37643762 Free PMC article.
-
Inhibitory effect and molecular mechanism of mesenchymal stem cells on NSCLC cells.Mol Cell Biochem. 2018 Apr;441(1-2):63-76. doi: 10.1007/s11010-017-3174-y. Epub 2017 Sep 8. Mol Cell Biochem. 2018. PMID: 28887716
References
-
- Altaner C. Prodrug cancer gene therapy. Cancer Lett. 2008;270:191–201. - PubMed
-
- Kim SK, Kim SU, Park IH, Bang JH, Aboody KS, Wang KC, et al. Human neural stem cells target experimental intracranial medulloblastoma and deliver a therapeutic gene leading to tumor regression. Clin Cancer Res. 2006;12:5550–5556. - PubMed
-
- Shimato S, Natsume A, Takeuchi H, Wakabayashi T, Fujii M, Ito M, et al. Human neural stem cells target and deliver therapeutic gene to experimental leptomeningeal medulloblastoma. Gene Ther. 2007;14:1132–1142. - PubMed
-
- Danks MK, Yoon KJ, Bush RA, Remack JS, Wierdl M, Tsurkan L, et al. Tumor-targeted enzyme/prodrug therapy mediates long-term disease-free survival of mice bearing disseminated neuroblastoma. Cancer Res. 2007;67:22–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

