The influence of bacterial diet on fat storage in C. elegans
- PMID: 19844570
- PMCID: PMC2760100
- DOI: 10.1371/journal.pone.0007545
The influence of bacterial diet on fat storage in C. elegans
Abstract
Background: The nematode Caenorhabditis elegans has emerged as an important model for studies of the regulation of fat storage. C. elegans feed on bacteria, and various strains of E. coli are commonly used in research settings. However, it is not known whether particular bacterial diets affect fat storage and metabolism.
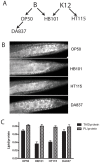
Methodology/principal findings: Fat staining of fixed nematodes, as well as biochemical analysis of lipid classes, revealed considerable differences in fat stores in C. elegans growing on four different E. coli strains. Fatty acid composition and carbohydrate levels differ in the E. coli strains examined in these studies, however these nutrient differences did not appear to have a causative effect on fat storage levels in worms. Analysis of C. elegans strains carrying mutations disrupting neuroendocrine and other fat-regulatory pathways demonstrated that the intensity of Nile Red staining of live worms does not correlate well with biochemical methods of fat quantification. Several neuroendocrine pathway mutants and eating defective mutants show higher or lower fat storage levels than wild type, however, these mutants still show differences in fat stores when grown on different bacterial strains. Of all the mutants tested, only pept-1 mutants, which lack a functional intestinal peptide transporter, fail to show differential fat stores. Furthermore, fatty acid analysis of triacylglycerol stores reveals an inverse correlation between total fat stores and the levels of 15-methylpalmitic acid, derived from leucine catabolism.
Conclusions: These studies demonstrate that nutritional cues perceived in the intestine regulate fat storage levels independently of neuroendocrine cues. The involvement of peptide transport and the accumulation of a fatty acid product derived from an amino acid suggest that specific peptides or amino acids may provide nutritional signals regulating fat metabolism and fat storage levels.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

