MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7
- PMID: 19844573
- PMCID: PMC2760121
- DOI: 10.1371/journal.pone.0007535
MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7
Abstract
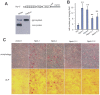
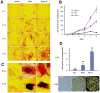
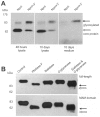
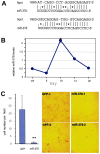
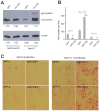
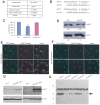
MicroRNAs (miRNAs) are small fragments of single-stranded RNA containing 18-24 nucleotides, and are generated from endogenous transcripts. MicroRNAs function in post-transcriptional gene silencing by targeting the 3'-untranslated region (UTR) of mRNAs, resulting in translational repression. We have developed a system to study the role of miRNAs in cell differentiation. We have found that one of the miRNAs tested in our system (miR-378, also called miR-378*) plays a role in modulating nephronectin-mediated differentiation in the osteoblastic cell line, MC3T3-E1. Nephronectin is an extracellular matrix protein, and we have demonstrated that its over-expression enhanced osteoblast differentiation and bone nodule formation. Furthermore, we found that the nephronectin 3'-untranslated region (3'UTR) contains a binding site for miR-378. Stable transfection of MC3T3-E1 cells with miR-378 inhibited cell differentiation. MC3T3-E1 cells stably transfected with nephronectin exhibited higher rates of differentiation and nodule formation as compared with cells transfected with nephronectin containing the 3'UTR in the early stages of development, suggesting that endogenous miR-378 is present and active. However, in the later stages of MC3T3-E1 development, the differentiation rates were opposite, with higher rates of differentiation and nodule formation in the cells over-expressing the 3'UTR of nephronectin. This appeared to be the consequence of competition between nephronectin and UDP-N-acetyl-alpha-D-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7 (GalNAc-T7 or GalNT7) for miR-378 binding, resulting in increased GalNT7 activity, which in turn lead to increased nephronectin glycosylation and product secretion, thereby resulting in a higher rate of osteoblast differentiation.
Conflict of interest statement
Figures



References
-
- Rubartelli A, Poggi A, Zocchi MR. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the alpha(v)beta3 integrin and requires intracellular and extracellular calcium. Eur J Immunol. 1997;27:1893–1900. - PubMed
-
- Hutvagner G, Zamore PD. A microRNA in a multiple-turnover RNAi enzyme complex. Science. 2002;297:2056–2060. - PubMed
-
- Lim LP, Glasner ME, Yekta S, Burge CB, Bartel DP. Vertebrate microRNA genes. Science. 2003;299:1540. - PubMed
-
- Lund E, Guttinger S, Calado A, Dahlberg JE, Kutay U. Nuclear export of microRNA precursors. Science. 2004;303:95–98. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

