Metagenomic approaches to natural products from free-living and symbiotic organisms
- PMID: 19844642
- PMCID: PMC2919151
- DOI: 10.1039/b817078a
Metagenomic approaches to natural products from free-living and symbiotic organisms
Abstract
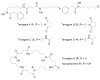
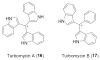
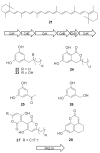
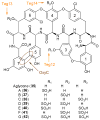
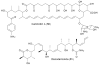
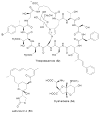
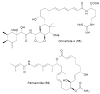
Bacterial cultivation has been a mainstay of natural products discovery for the past 80 years. However, the majority of bacteria are recalcitrant to culture, providing an untapped source for new natural products. Metagenomic analysis provides an alternative method to directly access the uncultivated genome for natural products research and for the discovery of novel, bioactive substances. Applications of metagenomics to diverse habitats, such as soils and the interior of animals, are described.
Figures
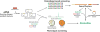
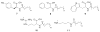
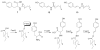
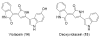
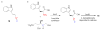
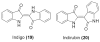
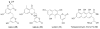
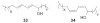

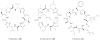
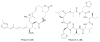
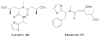
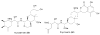

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

