Desensitization of endothelial P2Y1 receptors by PKC-dependent mechanisms in pressurized rat small mesenteric arteries
- PMID: 19845669
- PMCID: PMC2795227
- DOI: 10.1111/j.1476-5381.2009.00456.x
Desensitization of endothelial P2Y1 receptors by PKC-dependent mechanisms in pressurized rat small mesenteric arteries
Abstract
Background and purpose: Extracellular nucleotides play a crucial role in the regulation of vascular tone and blood flow. Stimulation of endothelial cell P2Y1 receptors evokes concentration-dependent full dilatation of resistance arteries. However, this GPCR can desensitize upon prolonged exposure to the agonist. Our aim was to determine the extent and nature of P2Y1 desensitization in isolated and pressurized rat small mesenteric arteries.
Experimental approach: The non-hydrolyzable selective P2Y1 agonist ADPbetaS (3 microM) was perfused through the lumen of arteries pressurized to 70 mmHg. Changes in arterial diameter and endothelial cell [Ca(2+)](i) were obtained in the presence and absence of inhibitors of protein kinase C (PKC).
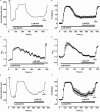
Key results: ADPbetaS evoked rapid dilatation to the maximum arterial diameter but faded over time to a much-reduced plateau closer to 35% dilatation. This appeared to be due to desensitization of the P2Y1 receptor, as subsequent endothelium-dependent dilatation to acetylcholine (1 microM) remained unaffected. Luminal treatment with the PKC inhibitors BIS-I (1 microM) or BIS-VIII (1 microM) tended to augment concentration-dependent dilatation to ADPbetaS (0.1-3 microM) and prevented desensitization. Another PKC inhibitor, Gö 6976 (1 microM), was less effective in preventing desensitization. Measurements of endothelial cell [Ca(2+)](i) in pressurized arteries confirmed the P2Y1 receptor but not M(3) muscarinic receptor desensitization.
Conclusions and implications: These data demonstrate for the first time the involvement of PKC in the desensitization of endothelial P2Y1 receptors in pressurized rat mesenteric arteries, which may have important implications in the control of blood flow by circulating nucleotides.
Figures





Similar articles
-
Spreading dilatation to luminal perfusion of ATP and UTP in rat isolated small mesenteric arteries.J Physiol. 2007 Jul 1;582(Pt 1):335-47. doi: 10.1113/jphysiol.2007.135202. Epub 2007 May 3. J Physiol. 2007. PMID: 17478526 Free PMC article.
-
Endothelium-dependent relaxation and endothelial hyperpolarization by P2Y receptor agonists in rat-isolated mesenteric artery.Br J Pharmacol. 2003 Jun;139(3):661-71. doi: 10.1038/sj.bjp.0705271. Br J Pharmacol. 2003. PMID: 12788826 Free PMC article.
-
Comparison of P2 receptor subtypes producing dilation in rat intracerebral arterioles.Stroke. 2003 Jun;34(6):1473-8. doi: 10.1161/01.STR.0000071527.10129.65. Epub 2003 May 1. Stroke. 2003. PMID: 12730558
-
Endothelial P2Y receptors induce hyperpolarisation of vascular smooth muscle by release of endothelium-derived hyperpolarising factor.Eur J Pharmacol. 1999 Jan 8;364(2-3):169-73. doi: 10.1016/s0014-2999(98)00848-6. Eur J Pharmacol. 1999. PMID: 9932720
-
Mechanisms underlying reduced P2Y(1) -receptor-mediated relaxation in superior mesenteric arteries from long-term streptozotocin-induced diabetic rats.Acta Physiol (Oxf). 2013 Jan;207(1):130-41. doi: 10.1111/j.1748-1716.2012.02469.x. Epub 2012 Aug 4. Acta Physiol (Oxf). 2013. PMID: 22759594
Cited by
-
Sustained release of prostaglandin E₂ in fibroblasts expressing ectopically cyclooxygenase 2 impairs P2Y-dependent Ca²⁺-mobilization.Mediators Inflamm. 2014;2014:832103. doi: 10.1155/2014/832103. Epub 2014 Aug 18. Mediators Inflamm. 2014. PMID: 25214717 Free PMC article.
-
Dynamics of inhibitory co-transmission, membrane potential and pacemaker activity determine neuromyogenic function in the rat colon.Pflugers Arch. 2014 Dec;466(12):2305-21. doi: 10.1007/s00424-014-1500-8. Pflugers Arch. 2014. PMID: 24658973
-
Ca2+ dependent but PKC independent signalling mediates UTP induced contraction of rat mesenteric arteries.J Smooth Muscle Res. 2015;51:58-69. doi: 10.1540/jsmr.51.58. J Smooth Muscle Res. 2015. PMID: 26447104 Free PMC article.
-
Connexin-43-dependent ATP release mediates macrophage activation during sepsis.Elife. 2019 Feb 8;8:e42670. doi: 10.7554/eLife.42670. Elife. 2019. PMID: 30735126 Free PMC article.
-
Function of Connexin-43 in Macrophages.Int J Mol Sci. 2021 Jan 30;22(3):1412. doi: 10.3390/ijms22031412. Int J Mol Sci. 2021. PMID: 33573367 Free PMC article. Review.
References
-
- Bailey CP, Smith FL, Kelly E, Dewey WL, Henderson G. How important is protein kinase C in mu-opioid receptor desensitization and morphine tolerance? Trends Pharmacol Sci. 2006;27:558–565. - PubMed
-
- Baurand A, Eckly A, Bari N, Léon C, Hechler B, Cazenave JP, et al. Desensitization of the platelet aggregation response to ADP: differential down-regulation of the P2Y1 and P2cyc receptors. Thromb Haemost. 2000;84:484–491. - PubMed
-
- Baurand A, Eckly A, Hechler B, Kauffenstein G, Galzi JL, Cazenave JP, et al. Differential regulation and relocalization of the platelet P2Y receptors after activation: a way to avoid loss of hemostatic properties? Mol Pharmacol. 2005;67:721–733. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

