A human coronavirus OC43 variant harboring persistence-associated mutations in the S glycoprotein differentially induces the unfolded protein response in human neurons as compared to wild-type virus
- PMID: 19846189
- PMCID: PMC7111944
- DOI: 10.1016/j.virol.2009.09.026
A human coronavirus OC43 variant harboring persistence-associated mutations in the S glycoprotein differentially induces the unfolded protein response in human neurons as compared to wild-type virus
Abstract
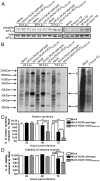
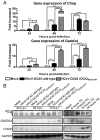
We have reported that human respiratory coronavirus OC43 (HCoV-OC43) is neurotropic and neuroinvasive in humans and mice, and that neurons are the primary target of infection in mice, leading to neurodegenerative disabilities. We now report that an HCoV-OC43 mutant harboring two persistence-associated S glycoprotein point mutations (H183R and Y241H), induced a stronger unfolded protein response (UPR) and translation attenuation in infected human neurons. There was a major contribution of the IRE1/XBP1 pathway, followed by caspase-3 activation and nuclear fragmentation, with no significant role of the ATF6 and eIF2-alpha/ATF4 pathways. Our results show the importance of discrete molecular viral S determinants in virus-neuronal cell interactions that lead to increased production of viral proteins and infectious particles, enhanced UPR activation, and increased cytotoxicity and cell death. As this mutant virus is more neurovirulent in mice, our results also suggest that two mutations in the S glycoprotein could eventually modulate viral neuropathogenesis.
Figures






Similar articles
-
Pivotal Role of Receptor-Interacting Protein Kinase 1 and Mixed Lineage Kinase Domain-Like in Neuronal Cell Death Induced by the Human Neuroinvasive Coronavirus OC43.J Virol. 2016 Dec 16;91(1):e01513-16. doi: 10.1128/JVI.01513-16. Print 2017 Jan 1. J Virol. 2016. PMID: 27795420 Free PMC article.
-
Glutamate excitotoxicity is involved in the induction of paralysis in mice after infection by a human coronavirus with a single point mutation in its spike protein.J Virol. 2011 Dec;85(23):12464-73. doi: 10.1128/JVI.05576-11. Epub 2011 Sep 28. J Virol. 2011. PMID: 21957311 Free PMC article.
-
Transcriptional induction of the human asparagine synthetase gene during the unfolded protein response does not require the ATF6 and IRE1/XBP1 arms of the pathway.Biochem J. 2009 Feb 1;417(3):695-703. doi: 10.1042/BJ20081706. Biochem J. 2009. PMID: 18840095 Free PMC article.
-
Testicular hyperthermia induces Unfolded Protein Response signaling activation in spermatocyte.Biochem Biophys Res Commun. 2013 May 17;434(4):861-6. doi: 10.1016/j.bbrc.2013.04.032. Epub 2013 Apr 20. Biochem Biophys Res Commun. 2013. PMID: 23611781
-
XBP1: the last two decades.Ann Rheum Dis. 2010 Jan;69 Suppl 1:i67-71. doi: 10.1136/ard.2009.119388. Ann Rheum Dis. 2010. PMID: 19995749 Review.
Cited by
-
Induction and modulation of the unfolded protein response during porcine deltacoronavirus infection.Vet Microbiol. 2022 Aug;271:109494. doi: 10.1016/j.vetmic.2022.109494. Epub 2022 Jun 14. Vet Microbiol. 2022. PMID: 35752087 Free PMC article.
-
Structural proteins of human coronaviruses: what makes them different?Front Cell Infect Microbiol. 2024 Dec 6;14:1458383. doi: 10.3389/fcimb.2024.1458383. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39711780 Free PMC article. Review.
-
How SARS-Cov-2 can involve the central nervous system. A systematic analysis of literature of the department of human neurosciences of Sapienza University, Italy.J Clin Neurosci. 2020 Sep;79:231-236. doi: 10.1016/j.jocn.2020.07.007. Epub 2020 Jul 7. J Clin Neurosci. 2020. PMID: 33070902 Free PMC article. Review.
-
SARS-CoV-2 Diverges from Other Betacoronaviruses in Only Partially Activating the IRE1α/XBP1 Endoplasmic Reticulum Stress Pathway in Human Lung-Derived Cells.mBio. 2022 Oct 26;13(5):e0241522. doi: 10.1128/mbio.02415-22. Epub 2022 Sep 20. mBio. 2022. PMID: 36125275 Free PMC article.
-
Neurotropism of SARS-CoV-2 and its neuropathological alterations: Similarities with other coronaviruses.Neurosci Biobehav Rev. 2020 Dec;119:184-193. doi: 10.1016/j.neubiorev.2020.10.012. Epub 2020 Oct 19. Neurosci Biobehav Rev. 2020. PMID: 33091416 Free PMC article. Review.
References
-
- Antony J.M., Ellestad K.K., Hammond R., Imaizumi K., Mallet F., Warren K.G., Power C. The human endogenous retrovirus envelope glycoprotein, syncytin-1, regulates neuroinflammation and its receptor expression in multiple sclerosis: a role for endoplasmic reticulum chaperones in astrocytes. J. Immunol. 2007;179:1210–1224. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

