Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells
- PMID: 19846341
- PMCID: PMC2787701
- DOI: 10.1016/j.it.2009.09.002
Neonatal immunity: faulty T-helpers and the shortcomings of dendritic cells
Abstract
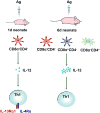
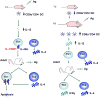
Immunity in the newborn is characterized by minimal T helper (Th)1 function but an excess of Th2 activity. Since Th1 lymphocytes are important to counter microbes and Th2 cells favor allergies, the newborn faces susceptibility to microbial infections and allergic reactions. Delayed maturation of certain dendritic cells leads to limited interleukin (IL)-12 production during the neonatal period. The Th2 cytokine locus of neonatal CD4(+) T cells is poised epigenetically for rapid and robust production of IL-4 and IL-13. Together, these circumstances lead to efficient differentiation of Th2 cells and the expression of an IL-4Ralpha/IL-13Ralpha1 heteroreceptor on Th1 cells. Upon re-challenge, Th2 cells rapidly produce IL-4 which utilizes the heteroreceptor to drive apoptosis of Th1 cells, thus yielding the Th2 bias of neonatal immunity.
Figures




References
-
- Billingham RE, et al. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. - PubMed
-
- Gammon G, et al. Neonatal T cell tolerance to minimal immunogenic peptides is caused by clonal inactivation. Nature. 1986;319:413–415. - PubMed
-
- Lawn J, et al. 4 million neonatal deaths: When? Where? Why? Lancet. 2005;365:9–18. - PubMed
-
- Prescott S, et al. Development of allergen-specific T-cell memory in atopic and normal children. Lancet. 1999;353:196–200. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

