The subthalamic nucleus in primary dystonia: single-unit discharge characteristics
- PMID: 19846625
- PMCID: PMC4073906
- DOI: 10.1152/jn.00544.2009
The subthalamic nucleus in primary dystonia: single-unit discharge characteristics
Abstract
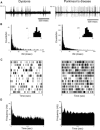
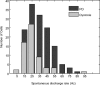
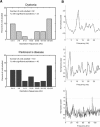
Most models of dystonia pathophysiology predict alterations of activity in the basal ganglia thalamocortical motor circuit. The globus pallidus interna (GPi) shows bursting and oscillatory neuronal discharge in both human dystonia and in animal models, but it is not clear which intrinsic basal ganglia pathways are implicated in this abnormal output. The subthalamic nucleus (STN) receives prominent excitatory input directly from cortical areas implicated in dystonia pathogenesis and inhibitory input from the external globus pallidus. The goal of this study was to elucidate the role of the STN in dystonia by analyzing STN neuronal discharge in patients with idiopathic dystonia. Data were collected in awake patients undergoing microelectrode recording for implantation of STN deep brain stimulation electrodes. We recorded 62 STN neurons in 9 patients with primary dystonia. As a comparison group, we recorded 143 STN neurons in 20 patients with Parkinson's disease (PD). Single-unit activity was discriminated off-line by principal component analysis and evaluated with respect to discharge rate, bursting, and oscillatory activity. The mean STN discharge rate in dystonia patients was 26.3 Hz (SD 13.6), which was lower than that in the PD patients (35.6 Hz, SD 15.2), but higher than published values for subjects without basal ganglia dysfunction. Oscillatory activity was found in both disorders, with a higher proportion of units oscillating in the beta range in PD. Bursting discharge was a prominent feature of both dystonia and PD, whereas sensory receptive fields were expanded in PD compared with dystonia. The STN firing characteristics, in conjunction with those previously published for GPi, suggest that bursting and oscillatory discharge in basal ganglia output may be transmitted via pathways involving the STN and provide a pathophysiologic rationale for STN as a surgical target in dystonia.
Figures

References
-
- Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci 12: 366–375, 1989 - PubMed
-
- Asanuma K, Ma Y, Okulski J, Dhawan V, Chaly T, Carbon M, Bressman SB, Eidelberg D. Decreased striatal D2 receptor binding in non-manifesting carriers of the DYT1 dystonia mutation. Neurology 64: 347–349, 2005 - PubMed
-
- Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol 72: 507–520, 1994 - PubMed
-
- Bevan MD, Hallworth NE, Baufreton J. GABAergic control of the subthalamic nucleus. Prog Brain Res 160: 173–188, 2007 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

