Small Cajal body-specific RNAs of Drosophila function in the absence of Cajal bodies
- PMID: 19846657
- PMCID: PMC2793299
- DOI: 10.1091/mbc.e09-09-0777
Small Cajal body-specific RNAs of Drosophila function in the absence of Cajal bodies
Abstract
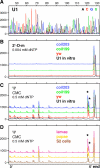
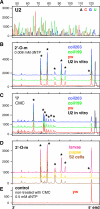
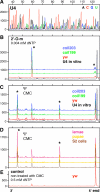
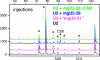
During their biogenesis small nuclear RNAs (snRNAs) undergo multiple covalent modifications that require guide RNAs to direct methylase and pseudouridylase enzymes to the appropriate nucleotides. Because of their localization in the nuclear Cajal body (CB), these guide RNAs are known as small CB-specific RNAs (scaRNAs). Using a fluorescent primer extension technique, we mapped the modified nucleotides in Drosophila U1, U2, U4, and U5 snRNAs. By fluorescent in situ hybridization (FISH) we showed that seven Drosophila scaRNAs are concentrated in easily detectable CBs. We used two assays based on Xenopus oocyte nuclei to demonstrate that three of these Drosophila scaRNAs do, in fact, function as guide RNAs. In flies null for the CB marker protein coilin, CBs are absent and there are no localized FISH signals for the scaRNAs. Nevertheless, biochemical experiments show that scaRNAs are present at normal levels and snRNAs are properly modified. Our experiments demonstrate that several scaRNAs are concentrated as expected in the CBs of wild-type Drosophila, but they function equally well in the nucleoplasm of mutant flies that lack CBs. We propose that the snRNA modification machinery is not limited to CBs, but is dispersed throughout the nucleoplasm of cells in general.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

