Exaggerated response of a vasopressin-enhanced green fluorescent protein transgene to nociceptive stimulation in the rat
- PMID: 19846706
- PMCID: PMC6665199
- DOI: 10.1523/JNEUROSCI.2624-09.2009
Exaggerated response of a vasopressin-enhanced green fluorescent protein transgene to nociceptive stimulation in the rat
Abstract
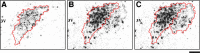
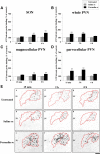
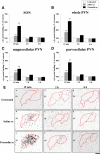
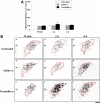
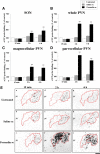
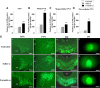
Nociceptive stimulation elicits neuroendocrine responses such as arginine vasopressin (AVP) release as well as activation of the hypothalamo-pituitary-adrenal axis. We have generated novel transgenic rats expressing an AVP-enhanced green fluorescent protein (eGFP) fusion gene, and we examined the effects of nociceptive stimulation on transgene expression in the hypothalamus after subcutaneous injection of saline or formalin into the bilateral hindpaws in these rats. We have assessed (1) AVP levels in plasma and the changes of eGFP mRNA and AVP heteronuclear RNA (hnRNA) in the supraoptic nucleus (SON) and the paraventricular nucleus (PVN) using in situ hybridization histochemistry, (2) gene expression changes in distinct magnocellular and parvocellular divisions of the PVN, (3) eGFP fluorescence in the SON, the PVN, the median eminence (ME), and the posterior pituitary gland (PP). Plasma AVP levels were significantly increased 15 min after formalin injection. In the same time period, the AVP hnRNA levels in the PVN were increased, especially in the parvocellular division of the PVN in formalin-injected rats. In the same region, eGFP mRNA levels after formalin injection were also significantly increased to a much greater extent than those of AVP hnRNA. The eGFP fluorescence in the SON, the PVN, the ME, and the PP was markedly increased in formalin-injected rats and especially increased in the parvocellular divisions of the PVN. Together, our results demonstrate robust and rapid changes in the expression of the AVP-eGFP transgene in the rat hypothalamus after acute nociceptive stimulation.
Figures
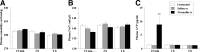
References
-
- Abbott FV, Melzack R, Samuel C. Morphine analgesia in tail-flick and formalin pain tests is mediated by different neural systems. Exp Neurol. 1982a;75:644–651. - PubMed
-
- Abbott FV, Melzack R, Leber BF. Morphine analgesia and tolerance in the tail-flick and formalin tests: dose-response relationships. Pharmacol Biochem Behav. 1982b;17:1213–1219. - PubMed
-
- Abram SE, Yaksh TL. Systemic lidocaine blocks nerve injury-induced hyperalgesia and nociceptor-driven spinal sensitization in the rat. Anesthesiology. 1994;80:383–391. discussion 325A. - PubMed
-
- Aguilera G, Rabadan-Diehl C. Regulation of vasopressin V1b receptors in the anterior pituitary gland of the rat. Exp Physiol. 2000;85:19S–26S. - PubMed
-
- Ahn DK, Kim KH, Ju JS, Kwon S, Park JS. Microinjection of arginine vasopressin into the central nucleus of amygdala suppressed nociceptive jaw opening reflex in freely moving rats. Brain Res Bull. 2001;55:117–121. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
