Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons
- PMID: 19846708
- PMCID: PMC2789299
- DOI: 10.1523/JNEUROSCI.3248-09.2009
Mrgprd-expressing polymodal nociceptive neurons innervate most known classes of substantia gelatinosa neurons
Abstract
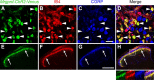
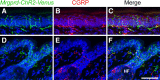
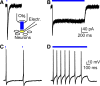
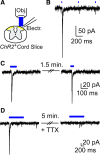
The Mas-related G-protein-coupled receptor D (Mrgprd) marks a distinct subset of sensory neurons that transmit polymodal nociceptive information from the skin epidermis to the substantia gelatinosa (SG, lamina II) of the spinal cord. Moreover, Mrgprd-expressing (Mrgprd(+)) neurons are required for the full expression of mechanical but not thermal nociception. While such anatomical and functional specificity suggests Mrgprd(+) neurons might synapse with specific postsynaptic targets in the SG, precisely how Mrgprd(+) neurons interface with spinal circuits is currently unknown. To study circuit connectivity, we genetically targeted the light-activated ion channel Channelrhodopsin-2-Venus (ChR2-Venus) to the Mrgprd locus. In these knock-in mice, ChR2-Venus was localized to nonpeptidergic Mrgprd(+) neurons and axons, while peptidergic CGRP(+) neurons were not significantly labeled. Dissociated Mrgprd(+) DRG neurons from mice expressing one or two copies of ChR2-Venus could be activated in vitro as evidenced by light-evoked currents and action potentials. In addition, illumination of Mrgprd-ChR2-Venus(+) axon terminals in spinal cord slices evoked EPSCs in half of all SG neurons. Within this subset, Mrgprd(+) neurons were monosynaptically connected to most known classes of SG neurons, including radial, tonic central, transient central, vertical, and antenna cells. This cellular diversity ruled out the possibility that Mrgprd(+) neurons innervate a dedicated class of SG neuron. Our findings set broad constraints on the types of spinal neurons that process afferent input from Mrgprd(+) polymodal nociceptors.
Figures
References
-
- Bessou P, Perl ER. Response of cutaneous sensory units with unmyelinated fibers to noxious stimuli. J Neurophysiol. 1969;32:1025–1043. - PubMed
-
- Boyden ES, Zhang F, Bamberg E, Nagel G, Deisseroth K. Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci. 2005;8:1263–1268. - PubMed
-
- Braz JM, Nassar MA, Wood JN, Basbaum AI. Parallel “pain” pathways arise from subpopulations of primary afferent nociceptor. Neuron. 2005;47:787–793. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
