Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas
- PMID: 19846796
- PMCID: PMC2797956
- DOI: 10.2337/dc09-1080
Closed-loop insulin delivery using a subcutaneous glucose sensor and intraperitoneal insulin delivery: feasibility study testing a new model for the artificial pancreas
Abstract
Objective: Attempts to build an artificial pancreas by using subcutaneous insulin delivery from a portable pump guided by an subcutaneous glucose sensor have encountered delays and variability of insulin absorption. We tested closed-loop intraperitoneal insulin infusion from an implanted pump driven by an subcutaneous glucose sensor via a proportional-integral-derivative (PID) algorithm.
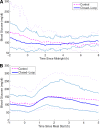
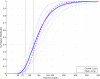
Research design and methods: Two-day closed-loop therapy (except for a 15-min pre-meal manual bolus) was compared with a 1-day control phase with intraperitoneal open-loop insulin delivery, according to randomized order, in a hospital setting in eight type 1 diabetic patients treated by implanted pumps. The percentage of time spent with blood glucose in the 4.4-6.6 mmol/l range was the primary end point. RESULTS During the closed-loop phases, the mean +/- SEM percentage of time spent with blood glucose in the 4.4-6.6 mmol/l range was significantly higher (39.1 +/- 4.5 vs. 27.7 +/- 6.2%, P = 0.05), and overall dispersion of blood glucose values was reduced among patients. Better closed-loop glucose control came from the time periods excluding the two early postprandial hours with a higher percentage of time in the 4.4-6.6 mmol/l range (46.3 +/- 5.3 vs. 28.6 +/- 7.4, P = 0.025) and lower mean blood glucose levels (6.9 +/- 0.3 vs. 7.9 +/- 0.6 mmol/l, P = 0.036). Time spent with blood glucose <3.3 mmol/l was low and similar for both investigational phases.
Conclusions: Our results demonstrate the feasibility of intraperitoneal insulin delivery for an artificial beta-cell and support the need for further study. Moreover, according to a semiautomated mode, the features of the pre-meal bolus in terms of timing and amount warrant further research.
Figures

References
-
- The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977– 986 - PubMed
-
- Nathan DM, Cleary PA, Backlund JY, Genuth SM, Lachin JM, Orchard TJ, Raskin P, Zinman B: Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research Group. Intensive diabetes management and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 2005; 353: 2643– 2653 - PMC - PubMed
-
- Renard E: Implantable closed-loop glucose-sensing and insulin delivery: the future for insulin pump therapy. Curr Opin Pharmacol 2002; 2: 708– 716 - PubMed
-
- The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997; 46: 271– 286 - PubMed
-
- Albisser AM, Leibel BS, Ewart TG, Davidovac Z, Botz CK, Zingg W, Schipper H, Gander R: Clinical control of diabetes by the artificial pancreas. Diabetes 1974; 23: 397– 404 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

