Adenosine blocks IFN-gamma-induced phosphorylation of STAT1 on serine 727 to reduce macrophage activation
- PMID: 19846878
- PMCID: PMC2916018
- DOI: 10.4049/jimmunol.0900331
Adenosine blocks IFN-gamma-induced phosphorylation of STAT1 on serine 727 to reduce macrophage activation
Abstract
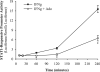
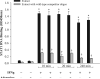
Macrophages are activated by IFN-gamma, a proinflammatory and proatherogenic cytokine that mediates its downstream effects primarily through STAT1. IFN-gamma signaling induces phosphorylation of two STAT1 residues: Tyr(701) (Y701), which facilitates dimerization, nuclear translocation, and DNA binding; and Ser(727) (S727), which enables maximal STAT1 transcription activity. Immunosuppressive molecules such as adenosine in the cellular microenvironment can reduce macrophage inflammatory and atherogenic functions through receptor-mediated signaling pathways. We hypothesized that adenosine achieves these protective effects by interrupting IFN-gamma signaling in activated macrophages. This investigation demonstrates that adding adenosine to IFN-gamma-stimulated murine RAW 264.7 and human THP-1 macrophages results in unique modulation of STAT1 serine and tyrosine phosphorylation events. We show that adenosine inhibits IFN-gamma-induced STAT1 S727 phosphorylation by >30% and phosphoserine-mediated transcriptional activity by 58% but has no effect on phosphorylation of Y701 or receptor-associated JAK tyrosine kinases. Inhibition of the adenosine A(3) receptor with a subtype-specific antagonist (MRS 1191 in RAW 264.7 cells and MRS 1220 in THP-1 cells) reverses this adenosine suppressive effect on STAT1 phosphoserine status by 25-50%. Further, RAW 264.7 A(3) receptor stimulation with Cl-IB-MECA reduces IFN-gamma-induced STAT1 transcriptional activity by 45% and STAT1-dependent gene expression by up to 80%. These data suggest that A(3) receptor signaling is key to adenosine-mediated STAT1 modulation and anti-inflammatory action in IFN-gamma-activated mouse and human macrophages. Because STAT1 plays a key role in IFN-gamma-induced inflammation and foam cell transformation, a better understanding of the mechanisms underlying STAT1 deactivation by adenosine may improve preventative and therapeutic approaches to vascular disease.
Figures





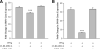



References
-
- Hansson GK, Robertson AK, Soderberg-Naucler C. Inflammation and atherosclerosis. Annu. Rev. Pathol. 2006;1:297–329. - PubMed
-
- Harvey EJ, Ramji DP. Interferon-γ and atherosclerosis: pro- or anti-atherogenic? Cardiovasc. Res. 2005;67:11–20. - PubMed
-
- Tedgui A, Mallat Z. Cytokines in atherosclerosis: pathogenic and regulatory pathways. Physiol. Rev. 2006;86:515–581. - PubMed
-
- Wuttge DM, Zhou X, Sheikine Y, Wagsater D, Stemme V, Hedin U, Stemme S, Hansson GK, Sirsjo A. CXCL16/SR-PSOX is an interferon-γ-regulated chemokine and scavenger receptor expressed in atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2004;24:750–755. - PubMed
-
- Panousis CG, Zuckerman SH. Regulation of cholesterol distribution in macrophage-derived foam cells by interferon-γ. J. Lipid Res. 2000;41:75–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

