An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization
- PMID: 19846886
- PMCID: PMC2834398
- DOI: 10.1182/blood-2009-07-235960
An in vitro model of differentiation of memory B cells into plasmablasts and plasma cells including detailed phenotypic and molecular characterization
Abstract
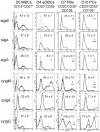
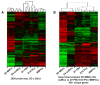
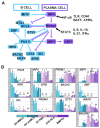
Human plasma cells (PCs) and their precursors play an essential role in humoral immune response but are rare and difficult to harvest. We report the generation of human syndecan-1(+) and immunoglobulin secreting PCs starting from memory B cells in a 3-step and 10-day (D) culture, including a 6-fold cell amplification. We report the detailed phenotypic and Affymetrix gene expression profiles of these in vitro PCs as well as of intermediate cells (activated B cells and plasmablasts) compared with memory B cells and bone marrow PCs, which is accessible through an open web ATLAS (http://amazonia.transcriptome.eu/). We show this B cell-to-PC differentiation to involve IRF4 and AICDA expressions in D4 activated B cells, decrease of PAX5 and BCL6 expressions, and increase in PRDM1 and XBP1 expressions in D7 plasmablasts and D10 PCs. It involves down-regulation of genes controlled by Pax5 and induction of genes controlled by Blimp-1 and XBP1 (unfold protein response). The detailed phenotype of D10 PCs resembles that of peripheral blood PCs detected after immunization of healthy donors. This in vitro model will facilitate further studies in PC biology. It will likewise be helpful to study PC dyscrasias, including multiple myeloma.
Figures




References
-
- Klein U, Dalla-Favera R. Germinal centres: role in B-cell physiology and malignancy. Nat Rev Immunol. 2008;8(1):22–33. - PubMed
-
- Batista FD, Harwood NE. The who, how and where of antigen presentation to B cells. Nat Rev Immunol. 2009;9(1):15–27. - PubMed
-
- Arce S, Luger E, Muehlinghaus G, et al. CD38 low IgG-secreting cells are precursors of various CD38 high-expressing plasma cell populations. J Leukoc Biol. 2004;75(6):1022–1028. - PubMed
-
- Gonzalez-Garcia I, Rodriguez-Bayona B, Mora-Lopez F, Campos-Caro A, Brieva JA. Increased survival is a selective feature of human circulating antigen-induced plasma cells synthesizing high-affinity antibodies. Blood. 2008;111(2):741–749. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

