Regulation by glutathionylation of isocitrate lyase from Chlamydomonas reinhardtii
- PMID: 19847013
- PMCID: PMC2794744
- DOI: 10.1074/jbc.M109.064428
Regulation by glutathionylation of isocitrate lyase from Chlamydomonas reinhardtii
Abstract
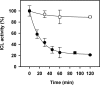
Post-translational modification of protein cysteine residues is emerging as an important regulatory and signaling mechanism. We have identified numerous putative targets of redox regulation in the unicellular green alga Chlamydomonas reinhardtii. One enzyme, isocitrate lyase (ICL), was identified both as a putative thioredoxin target and as an S-thiolated protein in vivo. ICL is a key enzyme of the glyoxylate cycle that allows growth on acetate as a sole source of carbon. The aim of the present study was to clarify the molecular mechanism of the redox regulation of Chlamydomonas ICL using a combination of biochemical and biophysical methods. The results clearly show that purified C. reinhardtii ICL can be inactivated by glutathionylation and reactivated by glutaredoxin, whereas thioredoxin does not appear to regulate ICL activity, and no inter- or intramolecular disulfide bond could be formed under any of the conditions tested. Glutathionylation of the protein was investigated by mass spectrometry analysis, Western blotting, and site-directed mutagenesis. The enzyme was found to be protected from irreversible oxidative inactivation by glutathionylation of its catalytic Cys(178), whereas a second residue, Cys(247), becomes artifactually glutathionylated after prolonged incubation with GSSG. The possible functional significance of this post-translational modification of ICL in Chlamydomonas and other organisms is discussed.
Figures





Similar articles
-
Lack of isocitrate lyase in Chlamydomonas leads to changes in carbon metabolism and in the response to oxidative stress under mixotrophic growth.Plant J. 2014 Feb;77(3):404-17. doi: 10.1111/tpj.12392. Epub 2014 Jan 7. Plant J. 2014. PMID: 24286363
-
Biochemical characterization of glutaredoxins from Chlamydomonas reinhardtii reveals the unique properties of a chloroplastic CGFS-type glutaredoxin.J Biol Chem. 2008 Apr 4;283(14):8868-76. doi: 10.1074/jbc.M709567200. Epub 2008 Jan 23. J Biol Chem. 2008. PMID: 18216016
-
The Deep Thioredoxome in Chlamydomonas reinhardtii: New Insights into Redox Regulation.Mol Plant. 2017 Aug 7;10(8):1107-1125. doi: 10.1016/j.molp.2017.07.009. Epub 2017 Jul 21. Mol Plant. 2017. PMID: 28739495
-
Protein glutathionylation in health and disease.Biochim Biophys Acta. 2013 May;1830(5):3165-72. doi: 10.1016/j.bbagen.2013.02.009. Epub 2013 Feb 15. Biochim Biophys Acta. 2013. PMID: 23416063 Review.
-
Respiratory-deficient mutants of the unicellular green alga Chlamydomonas: a review.Biochimie. 2014 May;100:207-18. doi: 10.1016/j.biochi.2013.10.006. Epub 2013 Oct 15. Biochimie. 2014. PMID: 24139906 Review.
Cited by
-
The Synechocystis PCC6803 MerA-like enzyme operates in the reduction of both mercury and uranium under the control of the glutaredoxin 1 enzyme.J Bacteriol. 2013 Sep;195(18):4138-45. doi: 10.1128/JB.00272-13. Epub 2013 Jul 12. J Bacteriol. 2013. PMID: 23852862 Free PMC article.
-
S-glutathionylation of ion channels: insights into the regulation of channel functions, thiol modification crosstalk, and mechanosensing.Antioxid Redox Signal. 2014 Feb 20;20(6):937-51. doi: 10.1089/ars.2013.5483. Epub 2013 Aug 20. Antioxid Redox Signal. 2014. PMID: 23834398 Free PMC article. Review.
-
Glutathionylation in the photosynthetic model organism Chlamydomonas reinhardtii: a proteomic survey.Mol Cell Proteomics. 2012 Feb;11(2):M111.014142. doi: 10.1074/mcp.M111.014142. Epub 2011 Nov 28. Mol Cell Proteomics. 2012. PMID: 22122882 Free PMC article.
-
Biochemical Validation of the Glyoxylate Cycle in the Cyanobacterium Chlorogloeopsis fritschii Strain PCC 9212.J Biol Chem. 2015 May 29;290(22):14019-30. doi: 10.1074/jbc.M115.648170. Epub 2015 Apr 13. J Biol Chem. 2015. PMID: 25869135 Free PMC article.
-
Chloroplasts lacking class I glutaredoxins are functional but show a delayed recovery of protein cysteinyl redox state after oxidative challenge.Redox Biol. 2024 Feb;69:103015. doi: 10.1016/j.redox.2023.103015. Epub 2023 Dec 28. Redox Biol. 2024. PMID: 38183796 Free PMC article.
References
-
- Michelet L., Zaffagnini M., Massot V., Keryer E., Vanacker H., Miginiac-Maslow M., Issakidis-Bourguet E., Lemaire S. D. (2006) Photosynth. Res. 89, 225–245 - PubMed
-
- Montrichard F., Alkhalfioui F., Yano H., Vensel W. H., Hurkman W. J., Buchanan B. B. (2009) J. Proteomics 72, 452–474 - PubMed
-
- Rouhier N., Lemaire S. D., Jacquot J. P. (2008) Annu. Rev. Plant Biol. 59, 143–166 - PubMed
-
- Dalle-Donne I., Rossi R., Colombo G., Giustarini D., Milzani A. (2009) Trends Biochem. Sci. 34, 85–96 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

