Involvement of RhoH GTPase in the development of B-cell chronic lymphocytic leukemia
- PMID: 19847197
- PMCID: PMC3869226
- DOI: 10.1038/leu.2009.217
Involvement of RhoH GTPase in the development of B-cell chronic lymphocytic leukemia
Abstract
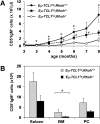
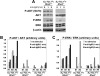
RhoH is a hematopoietic-specific, GTPase-deficient member of the Rho GTPase family that functions as a regulator of thymocyte development and T-cell receptor signaling by facilitating localization of zeta-chain-associated protein kinase 70 (ZAP70) to the immunological synapse. Here we investigated the function of RhoH in the B-cell lineage. B-cell receptor (BCR) signaling was intact in Rhoh(-/-) mice. Because RhoH interacts with ZAP70, which is a prognostic factor in B-cell chronic lymphocytic leukemia (CLL), we analyzed the mRNA levels of RhoH in primary human CLL cells and showed a 2.3-fold higher RhoH expression compared with normal B cells. RhoH expression in CLL positively correlated with the protein levels of ZAP70. Deletion of Rhoh in a murine model of CLL (Emu-TCL1(Tg) mice) significantly delayed the accumulation of CD5(+)IgM(+) leukemic cells in peripheral blood and the leukemic burden in the peritoneal cavity, bone marrow and spleen of Rhoh(-/-) mice compared with their Rhoh(+/+) counterparts. Phosphorylation of AKT and ERK in response to BCR stimulation was notably decreased in Emu-TCL1(Tg);Rhoh(-/-) splenocytes. These data suggest that RhoH has a function in the progression of CLL in a murine model and show RhoH expression is altered in human primary CLL samples.
Figures



References
-
- Chiorazzi N, Rai KR, Ferrarini M. Chronic lymphocytic leukemia. The New England journal of medicine. 2005 Feb 24;352(8):804–815. - PubMed
-
- Crespo M, Bosch F, Villamor N, Bellosillo B, Colomer D, Rozman M, et al. ZAP-70 expression as a surrogate for immunoglobulin-variable-region mutations in chronic lymphocytic leukemia. The New England journal of medicine. 2003 May 1;348(18):1764–1775. - PubMed
-
- Rassenti LZ, Huynh L, Toy TL, Chen L, Keating MJ, Gribben JG, et al. ZAP-70 compared with immunoglobulin heavy-chain gene mutation status as a predictor of disease progression in chronic lymphocytic leukemia. The New England journal of medicine. 2004 Aug 26;351(9):893–901. - PubMed
-
- Chen L, Widhopf G, Huynh L, Rassenti L, Rai KR, Weiss A, et al. Expression of ZAP-70 is associated with increased B-cell receptor signaling in chronic lymphocytic leukemia. Blood. 2002 Dec 15;100(13):4609–4614. - PubMed
-
- Chen L, Apgar J, Huynh L, Dicker F, Giago-McGahan T, Rassenti L, et al. ZAP-70 directly enhances IgM signaling in chronic lymphocytic leukemia. Blood. 2005 Mar 1;105(5):2036–2041. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

