The art of total synthesis through cascade reactions
- PMID: 19847336
- PMCID: PMC2766600
- DOI: 10.1039/b903290h
The art of total synthesis through cascade reactions
Abstract
The growing importance of cascade reactions reflects and imparts advances in the state of the art of organic synthesis and underscores the desire of synthetic chemists to achieve higher levels of elegance and efficiency. Besides their esthetic appeal, cascade processes offer economical and environmentally friendly means for generating molecular complexity. Because of their many advantages, these reactions have found numerous applications in the synthesis of complex molecules, both natural and designed. In this tutorial review, we highlight the design and execution of cascade reactions within the context of total synthesis as demonstrated with selected examples from these laboratories.
Figures








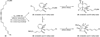



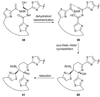






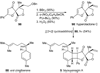

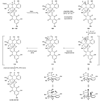
References
-
- Nicolaou KC, Edmonds DJ, Bulger PG. Angew. Chem., Int. Ed. 2006;45:7134. - PubMed
-
- Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem., Int. Ed. 2005;44:4442. - PubMed
-
- Nicolaou KC, Bulger PG, Sarlah D. Angew. Chem., Int. Ed. 2005;44:4490. - PubMed
-
- Bandaranayake WM, Banfield JE, St D, Black C, Fallon GD, Gatehouse BM. J. Chem. Soc., Chem. Commun. 1980:162.
-
- Bandaranayake WM, Banfield JE, St D, Black C. J. Chem. Soc., Chem. Commun. 1980:902.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

