The economies of synthesis
- PMID: 19847337
- PMCID: PMC2880393
- DOI: 10.1039/b821200g
The economies of synthesis
Abstract
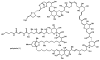
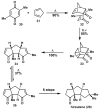
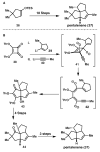
In this tutorial review the economies of synthesis are analysed from both detailed and macroscopic perspectives, using case-studies from complex molecule synthesis. Atom, step, and redox economy are more than philosophical constructs, but rather guidelines, which enable the synthetic chemist to design and execute an efficient synthesis. Students entering the field of synthesis might find this tutorial helpful for understanding the subtle differences between these economic principles and also see real-world situations where such principles are put into practice.
Figures

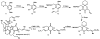

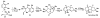
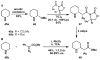
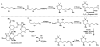
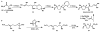
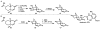



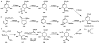

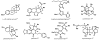
References
-
- Nicolaou KC, Montagnon T. Molecules That Changed The World. Wiley-VCH; Weinheim: 2008.
- Corey EJ, Czakó B, Kürti L. Molecules and Medicine. John Wiley & Sons, Inc.; Hoboken: 2007.
- Nicolaou KC, Vourloumis D, Winssinger N, Baran PS. Angew Chem. 2000;112:46–126. Angew. Chem., Int. Ed., 2000, 39, 44–126. - PubMed
- Nicolaou KC, Sorensen EJ. Classics in Total Synthesis. 1. Wiley-VCH; New York: 1996.
- Nicolaou KC, Snyder SA. Classics in Total Synthesis II. 1. Wiley-VCH; New York: 2003.
-
- Armstrong RW, Beau J-M, Cheon SH, Christ WJ, Fujioka H, Ham W-H, Hawkins LD, Jin H, Kang SH, Kishi Y, Martinelli MJ, McWhorther WW, Jr, Mizuno M, Nakata M, Stutz AE, Talamas FX, Taniguchi M, Tino JA, Ueda K, Uenishi J, White JB, Yonaga M. J Am Chem Soc. 1989;111:7530–7533.
- Suh EM, Kishi Y. J Am Chem Soc. 1994;116:11205–11206.
-
- Sheehan JC. The Enchanted Ring: The Untold Story of Penicillin. The MIT Press; Cambridge, MA, USA: 1982.
- Marx V. Chem Eng News. 2005;83(11):16–17.
-
- Hendrickson JB. J Am Chem Soc. 1975;97:5784–5800.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

