Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling
- PMID: 19847351
- PMCID: PMC2904069
- DOI: 10.1039/b821092f
Total synthesis of chiral biaryl natural products by asymmetric biaryl coupling
Abstract
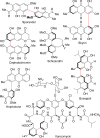
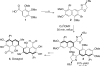
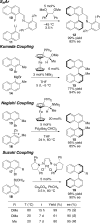
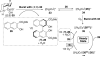
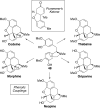
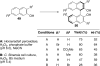
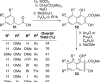
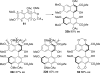
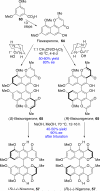
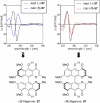
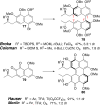
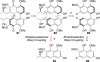
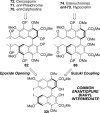
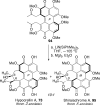
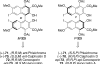
This tutorial review highlights the use of catalytic asymmetric 2-naphthol couplings in total synthesis. The types of chirality, chiral biaryl natural products, prior approaches to chiral biaryl natural products, and other catalytic asymmetric biaryl couplings are outlined. The three main categories of chiral catalysts for 2-naphthol coupling (Cu, V, Fe) are described with discussion of their limitations and advantages. Applications of the copper catalyzed couplings in biomimetic syntheses are discussed including nigerone, hypocrellin, calphostin D, phleichrome, and cercosporin.
Figures
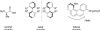


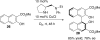








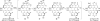



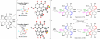


References
-
- Walsh PJ, Kozlowski MC. Fundamentals of Asymmetric Catalysis. University Science Books; Washington, D.C.: 2009.
-
- Meka L, Reha D, Havlas Z. J. Org. Chem. 2003;68:5677–5680. - PubMed
-
- Bringmann G, Günther C, Ochse M, Schupp O, Tasler S. Prog. Chem. Org. Nat. Prod. 2001;82:1–249. - PubMed
-
- Hassan J, Sevignon M, Gozzi C, Schulz E, Lemaire M. Chem. Rev. 2002;102:1359–1469. For a general review of biaryl bond forming methods. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

