Endothelin-1 as a neuropeptide: neurotransmitter or neurovascular effects?
- PMID: 19847673
- PMCID: PMC2821480
- DOI: 10.1007/s12079-009-0073-3
Endothelin-1 as a neuropeptide: neurotransmitter or neurovascular effects?
Abstract
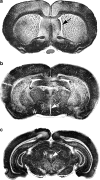
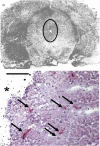
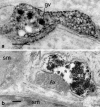
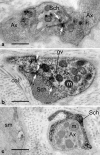
Endothelin-1 (ET-1) is an endothelium-derived peptide that also possesses potent mitogenic activity. There is also a suggestion the ET-1 is a neuropeptide, based mainly on its histological identification in both the central and peripheral nervous system in a number of species, including man. A neuropeptide role for ET-1 is supported by studies showing a variety of effects caused following its administration into different regions of the brain and by application to peripheral nerves. In addition there are studies proposing that ET-1 is implicated in a number of neural circuits where its transmitter affects range from a role in pain and temperature control to its action on the hypothalamo-neurosecretory system. While the effect of ET-1 on nerve tissue is beyond doubt, its action on nerve blood flow is often ignored. Here, we review data generated in a number of species and using a variety of experimental models. Studies range from those showing the distribution of ET-1 and its receptors in nerve tissue to those describing numerous neurally-mediated effects of ET-1.
Figures


References
-
- Aktan Ikiz ZA, Ucerler H, Bilge O. The anatomic features of the sural nerve with an emphasis on its clinical importance. Foot Ankle Int. 2005;26:560–567. - PubMed
-
- Barti-Mattera LN, Harwalkar S, Huges B, Wilkins PL, Almhanna K. Proliferative and morphological effects of endothelins in Schwann cells: roles of p38 mitogen-activated protein kinase and Ca2+-independenta phospholipase A2. J Neurochem. 2001;79:1136–1148. doi: 10.1046/j.1471-4159.2001.00642.x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

