A novel and efficient tool for locating and characterizing protein cavities and binding sites
- PMID: 19847777
- PMCID: PMC2811767
- DOI: 10.1002/prot.22608
A novel and efficient tool for locating and characterizing protein cavities and binding sites
Abstract
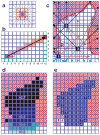
Systematic investigation of a protein and its binding site characteristics are crucial for designing small molecules that modulate protein functions. However, fundamental uncertainties in binding site interactions and insufficient knowledge of the properties of even well-defined binding pockets can make it difficult to design optimal drugs. Herein, we report the development and implementation of a cavity detection algorithm built with HINT toolkit functions that we are naming Vectorial Identification of Cavity Extents (VICE). This very efficient algorithm is based on geometric criteria applied to simple integer grid maps. In testing, we carried out a systematic investigation on a very diverse data set of proteins and protein-protein/protein-polynucleotide complexes for locating and characterizing the indentations, cavities, pockets, grooves, channels, and surface regions. Additionally, we evaluated a curated data set of unbound proteins for which a ligand-bound protein structures are also known; here the VICE algorithm located the actual ligand in the largest cavity in 83% of the cases and in one of the three largest in 90% of the cases. An interactive front-end provides a quick and simple procedure for locating, displaying and manipulating cavities in these structures. Information describing the cavity, including its volume and surface area metrics, and lists of atoms, residues, and/or chains lining the binding pocket, can be easily obtained and analyzed. For example, the relative cross-sectional surface area (to total surface area) of cavity openings in well-enclosed cavities is 0.06 +/- 0.04 and in surface clefts or crevices is 0.25 +/- 0.09. Proteins 2010. (c) 2009 Wiley-Liss, Inc.
Figures











References
-
- Sotriffer C, Klebe G. Identification and mapping of small-molecule binding sites in proteins: computational tools for structure-based drug design. Il Farmaco. 2002;57:243–251. - PubMed
-
- Campbell SJ, Gold ND, Jackson RM, Westhead DR. Ligand binding: functional site location, similarity and docking. Curr Opin Struct Biol. 2003;13:389–395. - PubMed
-
- Kleywegt GJ. Detection, delineation, measurement and display of cavities in macromolecular structures. Acta Crystallogr D. 1994;50:178–185. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

