Sleep-dependent improvement in visuomotor learning: a causal role for slow waves
- PMID: 19848357
- PMCID: PMC2753806
- DOI: 10.1093/sleep/32.10.1273
Sleep-dependent improvement in visuomotor learning: a causal role for slow waves
Abstract
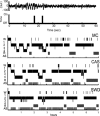
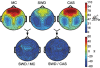
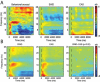
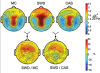
Study objectives: Sleep after learning often benefits memory consolidation, but the underlying mechanisms remain unclear. In previous studies, we found that learning a visuomotor task is followed by an increase in sleep slow wave activity (SWA, the electroencephalographic [EEG] power density between 0.5 and 4.5 Hz during non-rapid eye movement sleep) over the right parietal cortex. The SWA increase correlates with the postsleep improvement in visuomotor performance, suggesting that SWA may be causally responsible for the consolidation of visuomotor learning. Here, we tested this hypothesis by studying the effects of slow wave deprivation (SWD).
Design: After learning the task, subjects went to sleep, and acoustic stimuli were timed either to suppress slow waves (SWD) or to interfere as little as possible with spontaneous slow waves (control acoustic stimulation, CAS).
Setting: Sound-attenuated research room.
Participants: Healthy subjects (mean age 24.6 +/- 1.0 years; n = 9 for EEG analysis, n = 12 for behavior analysis; 3 women).
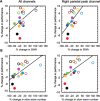
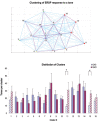
Measurements and results: Sleep time and efficiency were not affected, whereas SWA and the number of slow waves decreased in SWD relative to CAS. Relative to the night before, visuomotor performance significantly improved in the CAS condition (+5.93% +/- 0.88%) but not in the SWD condition (-0.77% +/- 1.16%), and the direct CAS vs SWD comparison showed a significant difference (P = 0.0007, n = 12, paired t test). Changes in visuomotor performance after SWD were correlated with SWA changes over right parietal cortex but not with the number of arousals identified using clinically established criteria, nor with any sign of "EEG lightening" identified using a novel automatic method based on event-related spectral perturbation analysis.
Conclusion: These results support a causal role for sleep slow waves in sleep-dependent improvement of visuomotor performance.
Figures



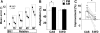

Comment in
-
Slow waves and learning: beyond correlations.Sleep. 2009 Oct;32(10):1253-4. doi: 10.1093/sleep/32.10.1253. Sleep. 2009. PMID: 19848353 Free PMC article. No abstract available.
References
-
- Born J, Rasch B, Gais S. Sleep to remember. Neuroscientist. 2006;12:410–424. - PubMed
-
- Giuditta A, Ambrosini MV, Montagnese P, et al. The sequential hypothesis of the function of sleep. Behav Brain Res. 1995;69:157–66. - PubMed
-
- Stickgold R, Whidbee D, Schirmer B, Patel V, Hobson JA. Visual discrimination task improvement: A multi-step process occurring during sleep. J Cogn Neurosci. 2000;12:246–54. - PubMed
-
- Plihal W, Born J. Effects of early and late nocturnal sleep on declarative and procedural memory. J Cogn Neurosci. 1997;9:534–47. - PubMed
-
- Steriade M. The corticothalamic system in sleep. Front Biosci. 2003;8:D878–99. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

