The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex
- PMID: 19850061
- PMCID: PMC2795133
- DOI: 10.1016/j.visres.2009.10.012
The influence of intrinsically-photosensitive retinal ganglion cells on the spectral sensitivity and response dynamics of the human pupillary light reflex
Abstract
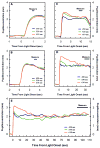
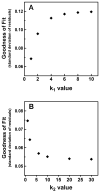
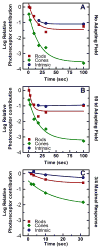
Historically, it was assumed that the light-evoked neural signals driving the human pupillary light reflex (PLR) originated exclusively from rod and cone photoreceptors. However, a novel melanopsin-containing photoreceptive cell class has recently been discovered in the mammalian retina. These intrinsically-photosensitive retinal ganglion cells (ipRGCs) project to the pretectum, the retinorecipient area of the brain responsible for the PLR. This study was therefore designed to examine the relative contribution of rod, cone and the melanopsin photoresponses of ipRGCs to the human PLR. We establish that the melanopsin photoresponse of ipRGCs contributes significantly to the maintenance of half maximal pupilloconstriction in response to light stimuli of 30s or longer, even at low photopic irradiances. Furthermore, we show that the melanopsin photoresponse contributes significantly to three-quarter maximal pupilloconstriction in response to light stimuli as short as 2s. We also demonstrate that cone photoresponses driving pupilloconstriction adapt considerably and contribute little after 30s, but rod photoresponses adapt less and contribute significantly to the maintenance of pupilloconstriction in response to steady-state light stimuli at irradiance levels which are below the threshold of the melanopsin photoresponse.
Figures

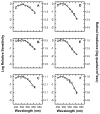

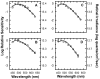
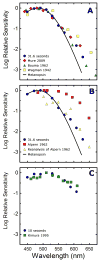
 ) shows good concordance with previous reports by Mure 2009 (
) shows good concordance with previous reports by Mure 2009 ( ), Bouma 1962 (
), Bouma 1962 ( ), and Wagman 1942 (
), and Wagman 1942 ( ) of the spectral sensitivity of the human PLR in response to steady-state light stimuli. Conversely, (B) Our data (
) of the spectral sensitivity of the human PLR in response to steady-state light stimuli. Conversely, (B) Our data ( ) is not in agreement with the report by Alpern 1962 (
) is not in agreement with the report by Alpern 1962 ( ) of the spectral sensitivity of steady-state PLR. However, when the data of Alpern 1962 is reanalyzed to correct for possible errors produced by incorrect baseline measurements (
) of the spectral sensitivity of steady-state PLR. However, when the data of Alpern 1962 is reanalyzed to correct for possible errors produced by incorrect baseline measurements ( ) (see section 4.6 for details), the results are similar to the results of the current study. (C) Our measured spectral sensitivity of the PLR for shorter stimulus durations (
) (see section 4.6 for details), the results are similar to the results of the current study. (C) Our measured spectral sensitivity of the PLR for shorter stimulus durations ( ) is also in good concordance with the spectral sensitivity measured by Kimura 1995 (
) is also in good concordance with the spectral sensitivity measured by Kimura 1995 ( ) for similar stimulus durations. All previously reported data were converted from corneal illuminance to retinal irradiance when necessary in order to facilitate the comparisons between studies. In panels A and B, the absorbance spectrum of melanopsin is represented by a Baylor nomogram (Baylor et al., 1987) with a lambda max at 483 nm.
) for similar stimulus durations. All previously reported data were converted from corneal illuminance to retinal irradiance when necessary in order to facilitate the comparisons between studies. In panels A and B, the absorbance spectrum of melanopsin is represented by a Baylor nomogram (Baylor et al., 1987) with a lambda max at 483 nm.References
-
- Adelson EH. Saturation and adaptation in the rod system. Vision Research. 1982;22(10):1299–1312. - PubMed
-
- Alpern M, Falls HF, Lee GB. The enigma of typical total monochromacy. Am J Ophthalmol. 1960;50:996–1012. - PubMed
-
- Alpern M, Ohba N. The effect of bleaching and backgrounds on pupil size. Vision Res. 1972;12(5):943–951. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

