Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical "disconnection"
- PMID: 19850138
- PMCID: PMC3725957
- DOI: 10.1016/j.neuroimage.2009.10.015
Altered white matter microstructure in the corpus callosum in Huntington's disease: implications for cortical "disconnection"
Abstract
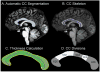
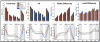
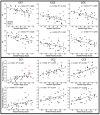
The corpus callosum (CC) is the major conduit for information transfer between the cerebral hemispheres and plays an integral role in relaying sensory, motor and cognitive information between homologous cortical regions. The majority of fibers that make up the CC arise from large pyramidal neurons in layers III and V, which project contra-laterally. These neurons degenerate in Huntington's disease (HD) in a topographically and temporally selective way. Since any focus of cortical degeneration could be expected to secondarily de-afferent homologous regions of cortex, we hypothesized that regionally selective cortical degeneration would be reflected in regionally selective degeneration of the CC. We used conventional T1-weighted, diffusion tensor imaging (DTI), and a modified corpus callosum segmentation scheme to examine the CC in healthy controls, huntingtin gene-carriers and symptomatic HD subjects. We measured mid-sagittal callosal cross-sectional thickness and several DTI parameters, including fractional anisotropy (FA), which reflects the degree of white matter organization, radial diffusivity, a suggested index of myelin integrity, and axial diffusivity, a suggested index of axonal damage of the CC. We found a topologically selective pattern of alterations in these measures in pre-manifest subjects that were more extensive in early symptomatic HD subjects and that correlated with performance on distinct cognitive measures, suggesting an important role for disrupted inter-hemispheric transfer in the clinical symptoms of HD. Our findings provide evidence for early degeneration of commissural pyramidal neurons in the neocortex, loss of cortico-cortical connectivity, and functional compromise of associative cortical processing.
Copyright 2009 Elsevier Inc. All rights reserved.
Conflict of interest statement
The authors have no financial interest in the research reported.
Figures
References
-
- Basser PJ, Pierpaoli C. Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B. 1996;111:209–219. - PubMed
-
- Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. - PubMed
-
- Browne SE. Mitochondria and Huntington’s disease pathogenesis: insight from genetic and chemical models. Ann N Y Acad Sci. 2008;1147:358–382. - PubMed
-
- Budde MD, Kim JH, Liang HF, Schmidt RE, Russell JH, Cross AH, Song SK. Toward accurate diagnosis of white matter pathology using diffusion tensor imaging. Magn Reson Med. 2007;57:688–695. - PubMed
-
- Cercignani M, Horsfield MA. The physical basis of diffusion-weighted MRI. J Neurol Sci. 2001;186(Suppl 1):S11–14. - PubMed
Publication types
MeSH terms
Grants and funding
- U24 RR021382/RR/NCRR NIH HHS/United States
- NR010827/NR/NINR NIH HHS/United States
- P41-RR14075/RR/NCRR NIH HHS/United States
- P01 NS058793/NS/NINDS NIH HHS/United States
- AG02238/AG/NIA NIH HHS/United States
- R01 EB001550/EB/NIBIB NIH HHS/United States
- P41 RR014075/RR/NCRR NIH HHS/United States
- R01 NS052585/NS/NINDS NIH HHS/United States
- K01 AG024898/AG/NIA NIH HHS/United States
- R01 NS042861/NS/NINDS NIH HHS/United States
- M01 RR001066/RR/NCRR NIH HHS/United States
- R01 RR16594-01A1/RR/NCRR NIH HHS/United States
- NS058792/NS/NINDS NIH HHS/United States
- K01AG024898/AG/NIA NIH HHS/United States
- R56 NS042861/NS/NINDS NIH HHS/United States
- R01 NR010827/NR/NINR NIH HHS/United States
- R01 RR016594/RR/NCRR NIH HHS/United States
- NS042861/NS/NINDS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

