Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2
- PMID: 19850877
- PMCID: PMC2765925
- DOI: 10.1073/pnas.0908853106
Homozygous loss of BHD causes early embryonic lethality and kidney tumor development with activation of mTORC1 and mTORC2
Abstract
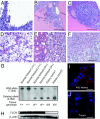
Germline mutations in the BHD/FLCN tumor suppressor gene predispose patients to develop renal tumors in the hamartoma syndrome, Birt-Hogg-Dubé (BHD). BHD encodes folliculin, a protein with unknown function that may interact with the energy- and nutrient-sensing AMPK-mTOR signaling pathways. To clarify BHD function in the mouse, we generated a BHD knockout mouse model. BHD homozygous null (BHD(d/d)) mice displayed early embryonic lethality at E5.5-E6.5, showing defects in the visceral endoderm. BHD heterozygous knockout (BHDd(/+)) mice appeared normal at birth but developed kidney cysts and solid tumors as they aged (median kidney-lesion-free survival = 23 months, median tumor-free survival = 25 months). As observed in human BHD kidney tumors, three different histologic types of kidney tumors developed in BHD(d/+) mice including oncocytic hybrid, oncocytoma, and clear cell with concomitant loss of heterozygosity (LOH), supporting a tumor suppressor function for BHD in the mouse. The PI3K-AKT pathway was activated in both human BHD renal tumors and kidney tumors in BHD(d/+) mice. Interestingly, total AKT protein was elevated in kidney tumors compared to normal kidney tissue, but without increased levels of AKT mRNA, suggesting that AKT may be regulated by folliculin through post translational or post-transcriptional modification. Finally, BHD inactivation led to both mTORC1 and mTORC2 activation in kidney tumors from BHD(d/+) mice and human BHD patients. These data support a role for PI3K-AKT pathway activation in kidney tumor formation caused by loss of BHD and suggest that inhibitors of both mTORC1 and mTORC2 may be effective as potential therapeutic agents for BHD-associated kidney cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




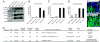
References
-
- Zbar B, et al. Risk of renal and colonic neoplasms and spontaneous pneumothorax in the Birt-Hogg-Dubé syndrome. Cancer Epidemiol Biomarkers Prev. 2002;11:393–400. - PubMed
-
- Toro JR, et al. Birt-Hogg-Dubé syndrome: A novel marker of kidney neoplasia. Arch Dermatol. 1999;135:1195–1202. - PubMed
-
- Birt AR, Hogg GR, Dubé WJ. Hereditary multiple fibrofolliculomas with trichodiscomas and acrochordons. Arch Dermatol. 1977;113:1674–1677. - PubMed
-
- Nickerson ML, et al. Mutations in a novel gene lead to kidney tumors, lung wall defects, and benign tumors of the hair follicle in patients with the Birt-Hogg-Dubé syndrome. Cancer Cell. 2002;2:157–164. - PubMed
-
- Pavlovich CP, et al. Renal tumors in the Birt-Hogg-Dubé syndrome. Am J Surg Pathol. 2002;26:1542–1552. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

