Powering through ribosome assembly
- PMID: 19850913
- PMCID: PMC2779681
- DOI: 10.1261/rna.1792109
Powering through ribosome assembly
Abstract
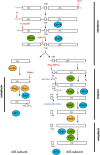
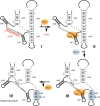
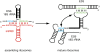
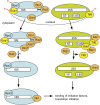
Ribosome assembly is required for cell growth in all organisms. Classic in vitro work in bacteria has led to a detailed understanding of the biophysical, thermodynamic, and structural basis for the ordered and correct assembly of ribosomal proteins on ribosomal RNA. Furthermore, it has enabled reconstitution of active subunits from ribosomal RNA and proteins in vitro. Nevertheless, recent work has shown that eukaryotic ribosome assembly requires a large macromolecular machinery in vivo. Many of these assembly factors such as ATPases, GTPases, and kinases hydrolyze nucleotide triphosphates. Because these enzymes are likely regulatory proteins, much work to date has focused on understanding their role in the assembly process. Here, we review these factors, as well as other sources of energy, and their roles in the ribosome assembly process. In addition, we propose roles of energy-releasing enzymes in the assembly process, to explain why energy is used for a process that occurs largely spontaneously in bacteria. Finally, we use literature data to suggest testable models for how these enzymes could be used as targets for regulation of ribosome assembly.
Figures

References
-
- Algire MA, Maag D, Lorsch JR. π Release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol Cell. 2005;20:251–262. - PubMed
-
- Alkemar G, Nygard O. Probing the secondary structure of expansion segment ES6 in 18S ribosomal RNA. Biochemistry. 2006;45:8067–8078. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
