Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit
- PMID: 19850918
- PMCID: PMC2794761
- DOI: 10.1074/jbc.M109.075523
Aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit
Abstract
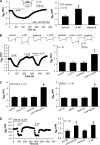
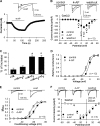
Aminopyridines such as 4-aminopyridine (4-AP) are widely used as voltage-activated K(+) (Kv) channel blockers and can improve neuromuscular function in patients with spinal cord injury, myasthenia gravis, or multiple sclerosis. Here, we present novel evidence that 4-AP and several of its analogs directly stimulate high voltage-activated Ca(2+) channels (HVACCs) in acutely dissociated neurons. 4-AP, 4-(aminomethyl)pyridine, 4-(methylamino)pyridine, and 4-di(methylamino)pyridine profoundly increased HVACC, but not T-type, currents in dissociated neurons from the rat dorsal root ganglion, superior cervical ganglion, and hippocampus. The widely used Kv channel blockers, including tetraethylammonium, alpha-dendrotoxin, phrixotoxin-2, and BDS-I, did not mimic or alter the effect of 4-AP on HVACCs. In HEK293 cells expressing various combinations of N-type (Cav2.2) channel subunits, 4-AP potentiated Ca(2+) currents primarily through the intracellular beta(3) subunit. In contrast, 4-AP had no effect on Cav3.2 channels expressed in HEK293 cells. Furthermore, blocking Kv channels did not mimic or change the potentiating effects of 4-AP on neurotransmitter release from sensory and motor nerve terminals. Thus, our findings challenge the conventional view that 4-AP facilitates synaptic and neuromuscular transmission by blocking Kv channels. Aminopyridines can directly target presynaptic HVACCs to potentiate neurotransmitter release independent of Kv channels.
Figures






Comment in
-
Changing the "channel": aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit.J Biol Chem. 2009 Dec 25;284(52):e99971. J Biol Chem. 2009. PMID: 20050319 Free PMC article. No abstract available.
-
Reported direct aminopyridine effects on voltage-gated calcium channels is a high-dose pharmacological off-target effect of no clinical relevance.J Biol Chem. 2018 Oct 12;293(41):16100. doi: 10.1074/jbc.L118.005425. J Biol Chem. 2018. PMID: 30315087 Free PMC article. No abstract available.
Similar articles
-
Changing the "channel": aminopyridines potentiate synaptic and neuromuscular transmission by targeting the voltage-activated calcium channel beta subunit.J Biol Chem. 2009 Dec 25;284(52):e99971. J Biol Chem. 2009. PMID: 20050319 Free PMC article. No abstract available.
-
Potentiation of high voltage-activated calcium channels by 4-aminopyridine depends on subunit composition.Mol Pharmacol. 2014 Dec;86(6):760-72. doi: 10.1124/mol.114.095505. Epub 2014 Sep 29. Mol Pharmacol. 2014. PMID: 25267719 Free PMC article.
-
Inhibition of neuronal degenerin/epithelial Na+ channels by the multiple sclerosis drug 4-aminopyridine.J Biol Chem. 2013 Mar 29;288(13):9418-27. doi: 10.1074/jbc.M112.449413. Epub 2013 Feb 12. J Biol Chem. 2013. PMID: 23404498 Free PMC article.
-
Voltage-activated potassium channels in mammalian neurons and their block by novel pharmacological agents.Gen Pharmacol. 1998 Jan;30(1):13-24. doi: 10.1016/s0306-3623(97)00034-7. Gen Pharmacol. 1998. PMID: 9457476 Review.
-
Ca2+ channels and synaptic transmission at the adult, neonatal, and P/Q-type deficient neuromuscular junction.Ann N Y Acad Sci. 2003 Sep;998:11-7. doi: 10.1196/annals.1254.003. Ann N Y Acad Sci. 2003. PMID: 14592858 Review.
Cited by
-
Distinct intrinsic and synaptic properties of pre-sympathetic and pre-parasympathetic output neurons in Barrington's nucleus.J Neurochem. 2013 Aug;126(3):338-48. doi: 10.1111/jnc.12290. Epub 2013 May 20. J Neurochem. 2013. PMID: 23647148 Free PMC article.
-
Blockade of fast A-type and TEA-sensitive potassium channels provide an antiparkinsonian effect in a 6-OHDA animal model.Neurosciences (Riyadh). 2017 Jan;22(1):44-50. doi: 10.17712/nsj.2017.1.20160266. Neurosciences (Riyadh). 2017. PMID: 28064330 Free PMC article.
-
Neurogenic Causes of Detrusor Underactivity.Curr Bladder Dysfunct Rep. 2015 Dec 1;10(4):325-331. doi: 10.1007/s11884-015-0331-6. Epub 2015 Sep 15. Curr Bladder Dysfunct Rep. 2015. PMID: 26715948 Free PMC article.
-
Dalfampridine: review of its efficacy in improving gait in patients with multiple sclerosis.J Cent Nerv Syst Dis. 2011 May 16;3:87-93. doi: 10.4137/JCNSD.S4868. Print 2011. J Cent Nerv Syst Dis. 2011. PMID: 23861641 Free PMC article.
-
A double-blind, randomized, controlled study of two dose strengths of dalfampridine extended release on walking deficits in ischemic stroke.Restor Neurol Neurosci. 2020;38(4):301-309. doi: 10.3233/RNN-201009. Restor Neurol Neurosci. 2020. PMID: 32651338 Free PMC article. Clinical Trial.
References
-
- Davis F. A., Stefoski D., Rush J. (1990) Ann. Neurol. 27, 186–192 - PubMed
-
- Hansebout R. R., Blight A. R., Fawcett S., Reddy K. (1993) J. Neurotrauma 10, 1–18 - PubMed
-
- Sanders D. B., Massey J. M., Sanders L. L., Edwards L. J. (2000) Neurology 54, 603–607 - PubMed
-
- Wirtz P. W., Verschuuren J. J., van Dijk J. G., de Kam M. L., Schoemaker R. C., van Hasselt J. G., Titulaer M. J., Tjaden U. R., den Hartigh J., van Gerven J. M. (2009) Clin. Pharmacol. Ther. 86, 44–48 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

