Designed auto-assembly of nanostreptabodies for rapid tissue-specific targeting in vivo
- PMID: 19850928
- PMCID: PMC2804220
- DOI: 10.1074/jbc.M109.061838
Designed auto-assembly of nanostreptabodies for rapid tissue-specific targeting in vivo
Abstract
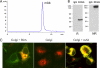
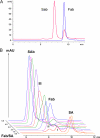
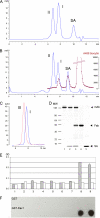
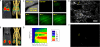
Molecular medicine can benefit greatly from antibodies that deliver therapeutic and imaging agents to select organs and diseased tissues. Yet the development of complex and defined composite nanostructures remains a challenge that requires both designed stoichiometric assembly and superior in vivo testing ability. Here, we generate nanostructures called nanostreptabodies by controlled sequential assembly of biotin-engineered antibody fragments on a streptavidin scaffold with a defined capacity for additional biotinylated payloads such as other antibodies to create bispecific antibodies as well as organic and non-organic moieties. When injected intravenously, these novel and stable nanostructures exhibit exquisite targeting with tissue-specific imaging and delivery, including rapid transendothelial transport that enhances tissue penetration. This "tinkertoy construction" strategy provides a very flexible and efficient way to link targeting vectors with reporter and/or effector agents, thereby providing virtually endless combinations potentially useful for multipurpose molecular and functional imaging in vivo as well as therapies.
Figures



References
-
- Coloma M. J., Morrison S. L. (1997) Nat. Biotechnol. 15, 159–163 - PubMed
-
- Lee H. S., Shu L., De Pascalis R., Giuliano M., Zhu M., Padlan E. A., Hand P. H., Schlom J., Hong H. J., Kashmiri S. V. (1999) Mol. Immunol. 36, 61–71 - PubMed
-
- Marvin J. S., Zhu Z. (2005) Acta. Pharmacol. Sin. 26, 649–658 - PubMed
-
- Plückthun A., Pack P. (1997) Immunotechnology 3, 83–105 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

