Reduction of beta-amyloid levels by novel protein kinase C(epsilon) activators
- PMID: 19850930
- PMCID: PMC2787312
- DOI: 10.1074/jbc.M109.016683
Reduction of beta-amyloid levels by novel protein kinase C(epsilon) activators
Abstract
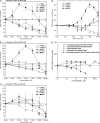
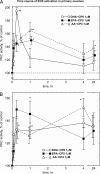
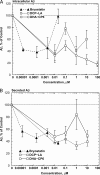
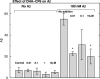
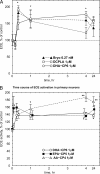
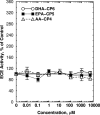
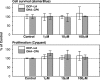
Isoform-specific protein kinase C (PKC) activators may be useful as therapeutic agents for the treatment of Alzheimer disease. Three new epsilon-specific PKC activators, made by cyclopropanation of polyunsaturated fatty acids, have been developed. These activators, AA-CP4, EPA-CP5, and DHA-CP6, activate PKCepsilon in a dose-dependent manner. Unlike PKC activators that bind to the 1,2-diacylglycerol-binding site, such as bryostatin and phorbol esters, which produce prolonged down-regulation, the new activators produced sustained activation of PKC. When applied to cells expressing human APPSwe/PS1delta, which produce large quantities of beta-amyloid peptide (Abeta), DCP-LA and DHA-CP6 reduced the intracellular and secreted levels of Abeta by 60-70%. In contrast to the marked activation of alpha-secretase produced by PKC activators in fibroblasts, the PKC activators produced only a moderate and transient activation of alpha-secretase in neuronal cells. However, they activated endothelin-converting enzyme to 180% of control levels, suggesting that the Abeta-lowering ability of these PKCepsilon activators is caused by increasing the rate of Abeta degradation by endothelin-converting enzyme and not by activating nonamyloidogenic amyloid precursor protein metabolism.
Figures


Similar articles
-
PKC ε activation prevents synaptic loss, Aβ elevation, and cognitive deficits in Alzheimer's disease transgenic mice.J Neurosci. 2011 Jan 12;31(2):630-43. doi: 10.1523/JNEUROSCI.5209-10.2011. J Neurosci. 2011. PMID: 21228172 Free PMC article.
-
Bryostatin-1 vs. TPPB: dose-dependent APP processing and PKC-α, -δ, and -ε isoform activation in SH-SY5Y neuronal cells.J Mol Neurosci. 2012 Sep;48(1):234-44. doi: 10.1007/s12031-012-9816-3. Epub 2012 Jun 15. J Mol Neurosci. 2012. PMID: 22700373 Free PMC article.
-
Evaluation of Toxic Amyloid 42 Oligomers in Rat Primary Cerebral Cortex Cells and Human iPS-derived Neurons Treated with 10-Me-Aplog-1, a New PKC Activator.Int J Mol Sci. 2020 Feb 11;21(4):1179. doi: 10.3390/ijms21041179. Int J Mol Sci. 2020. PMID: 32053979 Free PMC article.
-
Powering Amyloid Beta Degrading Enzymes: A Possible Therapy for Alzheimer's Disease.Neurochem Res. 2019 Jun;44(6):1289-1296. doi: 10.1007/s11064-019-02756-x. Epub 2019 Feb 26. Neurochem Res. 2019. PMID: 30806879 Review.
-
Role of the APP non-amyloidogenic signaling pathway and targeting alpha-secretase as an alternative drug target for treatment of Alzheimer's disease.Curr Med Chem. 2007;14(27):2848-64. doi: 10.2174/092986707782360060. Curr Med Chem. 2007. PMID: 18045131 Review.
Cited by
-
Protein Kinase Cϵ (PKCϵ) Promotes Synaptogenesis through Membrane Accumulation of the Postsynaptic Density Protein PSD-95.J Biol Chem. 2016 Aug 5;291(32):16462-76. doi: 10.1074/jbc.M116.730440. Epub 2016 Jun 21. J Biol Chem. 2016. PMID: 27330081 Free PMC article.
-
Apolipoprotein E3 (ApoE3) but not ApoE4 protects against synaptic loss through increased expression of protein kinase C epsilon.J Biol Chem. 2012 May 4;287(19):15947-58. doi: 10.1074/jbc.M111.312710. Epub 2012 Mar 17. J Biol Chem. 2012. PMID: 22427674 Free PMC article.
-
Bryostatin-1 alleviates experimental multiple sclerosis.Proc Natl Acad Sci U S A. 2018 Feb 27;115(9):2186-2191. doi: 10.1073/pnas.1719902115. Epub 2018 Feb 12. Proc Natl Acad Sci U S A. 2018. PMID: 29440425 Free PMC article.
-
Effects of corticotrophin-releasing factor receptor 1 antagonists on amyloid-β and behavior in Tg2576 mice.Psychopharmacology (Berl). 2014 Dec;231(24):4711-22. doi: 10.1007/s00213-014-3629-8. Epub 2014 May 27. Psychopharmacology (Berl). 2014. PMID: 24862368 Free PMC article.
-
A role for the PKC signaling system in the pathophysiology and treatment of mood disorders: involvement of a functional imbalance?Mol Neurobiol. 2011 Dec;44(3):407-19. doi: 10.1007/s12035-011-8210-4. Epub 2011 Oct 5. Mol Neurobiol. 2011. PMID: 21983961 Review.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

