Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone
- PMID: 19851510
- PMCID: PMC2762317
- DOI: 10.1371/journal.pone.0007583
Critical role of activating transcription factor 4 in the anabolic actions of parathyroid hormone in bone
Abstract
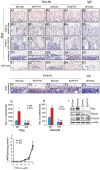
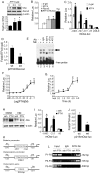
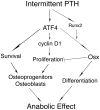
Parathyroid hormone (PTH) is a potent anabolic agent for the treatment of osteoporosis. However, its mechanism of action in osteoblast and bone is not well understood. In this study, we show that the anabolic actions of PTH in bone are severely impaired in both growing and adult ovariectomized mice lacking bone-related activating transcription factor 4 (ATF4). Our study demonstrates that ATF4 deficiency suppresses PTH-stimulated osteoblast proliferation and survival and abolishes PTH-induced osteoblast differentiation, which, together, compromise the anabolic response. We further demonstrate that the PTH-dependent increase in osteoblast differentiation is correlated with ATF4-dependent up-regulation of Osterix. This regulation involves interactions of ATF4 with a specific enhancer sequence in the Osterix promoter. Furthermore, actions of PTH on Osterix require this same element and are associated with increased binding of ATF4 to chromatin. Taken together these experiments establish a fundamental role for ATF4 in the anabolic actions of PTH on the skeleton.
Conflict of interest statement
Figures

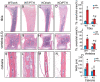
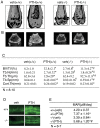
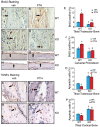
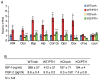
References
-
- Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344:1434–1441. - PubMed
-
- Demiralp B, Chen HL, Koh AJ, Keller ET, McCauley LK. Anabolic actions of parathyroid hormone during bone growth are dependent on c-fos. Endocrinology. 2002;143:4038–4047. - PubMed
-
- Iida-Klein A, Zhou H, Lu SS, Levine LR, Ducayen-Knowles M, et al. Anabolic action of parathyroid hormone is skeletal site specific at the tissue and cellular levels in mice. J Bone Miner Res. 2002;17:808–816. - PubMed
-
- Iida-Klein A, Lu SS, Kapadia R, Burkhart M, Moreno A, et al. Short-term continuous infusion of human parathyroid hormone 1-34 fragment is catabolic with decreased trabecular connectivity density accompanied by hypercalcemia in C57BL/J6 mice. J Endocrinol. 2005;186:549–557. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

