Gastrointestinal tolerability with ibandronate after previous weekly bisphosphonate treatment
- PMID: 19851511
- PMCID: PMC2762360
- DOI: 10.2147/cia.s5637
Gastrointestinal tolerability with ibandronate after previous weekly bisphosphonate treatment
Abstract
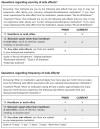
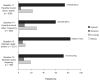
Data from two open-label trials (PRIOR and CURRENT) of women with postmenopausal osteoporosis or osteopenia were evaluated to assess whether monthly oral and quarterly intravenous (IV) ibandronate dosing improved self-reported gastrointestinal (GI) tolerability for patients who had previously experienced GI irritation with bisphosphonate (BP) use. In PRIOR, women who had discontinued daily or weekly BP treatment due to GI intolerance received monthly oral or quarterly IV ibandronate for 12 months. The CURRENT subanalysis included women receiving weekly BP treatment who switched to monthly oral ibandronate for six months. GI symptom severity and frequency were assessed using the Osteoporosis Patient Satisfaction Questionnaire. In PRIOR, mean GI tolerability scores increased significantly at month 1 from screening for both treatment groups (oral: 79.3 versus 54.1; IV: 84.4 versus 51.0; p < 0.001 for both). Most patients reported improvement in GI symptom severity and frequency from baseline at all post-screening assessments (>90% at Month 10). In the CURRENT subanalysis >60% of patients reported improvements in heartburn or acid reflux and >70% indicated improvement in other stomach upset at month 6. Postmenopausal women with GI irritability with daily or weekly BPs experienced improvement in symptoms with extended dosing monthly or quarterly ibandronate compared with baseline.
Keywords: bisphosphonate; gastrointestinal; ibandronate; osteoporosis.
Figures

References
-
- Eastell R. Treatment of postmenopausal osteoporosis. N Engl J Med. 1998;338(11):736–746. - PubMed
-
- Guyatt GH, Cranney A, Griffith L, et al. Summary of meta-analyses of therapies for postmenopausal osteoporosis and the relationship between bone density and fractures. Endocrinol Metab Clin North Am. 2002;31(3):659–679. - PubMed
-
- Black DM, Thompson DE. The effect of alendronate therapy on osteoporotic fracture in the vertebral fracture arm of the Fracture Intervention Trial. Int J Clin Pract Suppl. 1999;101:46–50. - PubMed
-
- Chesnut CH, Skag A, Christiansen C, et al. Effects of oral ibandronate administered daily or intermittently on fracture risk in postmenopausal osteoporosis. J Bone Miner Res. 2004;19(8):1241–1249. - PubMed
-
- Harris ST, Watts NB, Genant HK, et al. Effects of risedronate treatment on vertebral and nonvertebral fractures in women with postmenopausal osteoporosis: a randomized controlled trial. Vertebral Efficacy With Risedronate Therapy (VERT) Study Group. JAMA. 1999;282(14):1344–1352. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

