C-H bond functionalization via hydride transfer: direct coupling of unactivated alkynes and sp(3) C-H bonds catalyzed by platinum tetraiodide
- PMID: 19852462
- PMCID: PMC2971677
- DOI: 10.1021/ja906480w
C-H bond functionalization via hydride transfer: direct coupling of unactivated alkynes and sp(3) C-H bonds catalyzed by platinum tetraiodide
Abstract
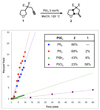
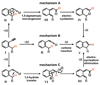
We report a catalytic intramolecular coupling between terminal unactivated alkynes and sp(3) C-H bonds via through-space hydride transfer (HT-cyclization of alkynes). This method enables one-step preparation of complex heterocyclic compounds by alpha-alkenylation of readily available cyclic ethers and amines. We show that PtI(4) is an effective Lewis acid catalyst for the activation of terminal alkynes for hydride attack and subsequent C-C bond formation. In addition, we have shown that the activity of neutral platinum salts (PtX(n)) can be modulated by the halide ligands. This modulation in turn allows for fine-tuning of the platinum center reactivity to match the reactivity and stability of selected substrates and products.
Figures
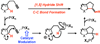


References
-
- Godula K, Sames D. Science. 2006;312:67. - PubMed
-
-
For other recent developments in the area of HT-cyclization, see: Zhang C, Murarka S, Seidel D. J. Org. Chem. 2009;74:419. Ruble JC, Hurd AR, Johnson TA, Sherry DA, Barbachyn MR, Toogood PL, Bundy GL, Graber DR, Kamilar GM. J. Am. Chem. Soc. 2009;131:3991. Zhang C, De CK, Mal R, Seidel D. J. Am. Chem. Soc. 2008;130:416.
-
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

