AT-101, a small molecule inhibitor of anti-apoptotic Bcl-2 family members, activates the SAPK/JNK pathway and enhances radiation-induced apoptosis
- PMID: 19852810
- PMCID: PMC2771029
- DOI: 10.1186/1748-717X-4-47
AT-101, a small molecule inhibitor of anti-apoptotic Bcl-2 family members, activates the SAPK/JNK pathway and enhances radiation-induced apoptosis
Abstract
Background: Gossypol, a naturally occurring polyphenolic compound has been identified as a small molecule inhibitor of anti-apoptotic Bcl-2 family proteins. It induces apoptosis in a wide range of tumor cell lines and enhances chemotherapy- and radiation-induced cytotoxicity both in vitro and in vivo. Bcl-2 and related proteins are important inhibitors of apoptosis and frequently overexpressed in human tumors. Increased levels of these proteins confer radio- and chemoresistance and may be associated with poor prognosis. Consequently, inhibition of the anti-apoptotic functions of Bcl-2 family members represents a promising strategy to overcome resistance to anticancer therapies.
Methods: We tested the effect of (-)-gossypol, also denominated as AT-101, radiation and the combination of both on apoptosis induction in human leukemic cells, Jurkat T and U937. Because activation of the SAPK/JNK pathway is important for apoptosis induction by many different stress stimuli, and Bcl-X(L) is known to inhibit activation of SAPK/JNK, we also investigated the role of this signaling cascade in AT-101-induced apoptosis using a pharmacologic and genetic approach.
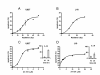
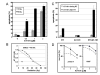
Results: AT-101 induced apoptosis in a time- and dose-dependent fashion, with ED50 values of 1.9 and 2.4 microM in Jurkat T and U937 cells, respectively. Isobolographic analysis revealed a synergistic interaction between AT-101 and radiation, which also appeared to be sequence-dependent. Like radiation, AT-101 activated SAPK/JNK which was blocked by the kinase inhibitor SP600125. In cells overexpressing a dominant-negative mutant of c-Jun, AT-101-induced apoptosis was significantly reduced.
Conclusion: Our data show that AT-101 strongly enhances radiation-induced apoptosis in human leukemic cells and indicate a requirement for the SAPK/JNK pathway in AT-101-induced apoptosis. This type of apoptosis modulation may overcome treatment resistance and lead to the development of new effective combination therapies.
Figures



References
-
- Belka C, Jendrossek V, Pruschy M, Vink S, Verheij M, Budach W. Apoptosis-modulating agents in combination with radiotherapy-current status and outlook. Int J Radiat Oncol Biol Phys. 2004;58:542–554. - PubMed
-
- Olopade OI, Adeyanju MO, Safa AR, Hagos F, Mick R, Thompson CB, Recant WM. Overexpression of BCL-x protein in primary breast cancer is associated with high tumor grade and nodal metastases. Cancer J Sci Am. 1997;3:230–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

