Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study
- PMID: 19853287
- PMCID: PMC4470610
- DOI: 10.1016/j.ygyno.2009.09.006
Prognostic factors for response to cisplatin-based chemotherapy in advanced cervical carcinoma: a Gynecologic Oncology Group Study
Abstract
Purpose: Cisplatin-based combination chemotherapy is considered standard treatment for advanced/recurrent cervical carcinoma; however, the majority of patients do not respond. This study was undertaken to identify the prognostic factors and develop a model predictive of (non-) response to chemotherapy.
Methods: Four-hundred twenty-eight patients with advanced cervical cancer who received a cisplatin-containing combination in three Gynecologic Oncology Group (GOG) protocols (110, 169 and 179) were evaluated for baseline clinical characteristics and multivariate analysis was conducted to identify factors independently prognostic predictive of response using a Logistic regression model. A predictive model was developed and externally validated using an independent GOG protocol (149) data.
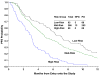
Results: Multivariate analysis identified five factors (African-American, performance status [PS] >0, pelvic disease, prior radiosensitizer and time interval from diagnosis to first recurrence <1 year) independently prognostic of poor response. A simple prognostic index was derived based on the total number of risk factors. When patients were classified into three risk groups (low risk: 0-1 factor; mid risk: 2-3 factors; high risk: 4-5 factors), patients with 4-5 risk factors were estimated to have a response rate of only 13%, and median progression-free and overall survival of 2.8 months and 5.5 months, respectively. The accuracy of the index was supported by both internal and external datasets.
Conclusions: A simple index based on five prognostic factors may have utility in clinical practice to identify the women who are not likely to respond to the cisplatin-containing regimens. This subgroup of patients should be considered for non-cisplatin chemotherapy or investigational trials.
Figures


References
-
- American Cancer Society. Cancer Facts & Figures 2008. Atlanta: American Cancer Society; 2008.
-
- Moore DH. Chemotherapy for advanced, recurrent, and metastatic cervical cancer. J Natl Compr Canc Netw. 2008;6:53–7. - PubMed
-
- Omura GA, Blessing JA, Vaccarello L, et al. A randomized trial of cisplatin versus cisplatin plus mitolactol versus cisplatin plus ifosfamide in advanced squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 1997;15:165–71. - PubMed
-
- Bloss JD, Blessing JA, Behrens BC, et al. Randomized trial of cisplatin and ifosfamide with or without bleomycin in squamous carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2002;20:1832–7. - PubMed
-
- Moore DH, Blessing JA, McQuellon RP, et al. Phase III study of cisplatin with or without paclitaxel in stage IVB, recurrent, or persistent squamous cell carcinoma of the cervix: a Gynecologic Oncology Group study. J Clin Oncol. 2004;22:3113–9. - PubMed

