Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds
- PMID: 19853296
- PMCID: PMC4367859
- DOI: 10.1016/j.biomaterials.2009.10.010
Doxycycline loaded poly(ethylene glycol) hydrogels for healing vesicant-induced ocular wounds
Abstract
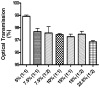
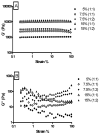
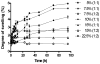
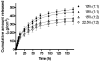
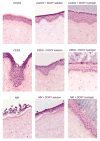
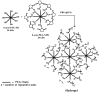
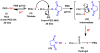
Half mustard (CEES) and nitrogen mustard (NM) are commonly used surrogates and vesicant analogs of the chemical warfare agent sulfur mustard. In the current study, in situ forming poly(ethylene glycol) (PEG)-based doxycycline hydrogels are developed and evaluated for their wound healing efficacy in CEES and NM-exposed rabbit corneas in organ culture. The hydrogels, characterized by UV-Vis spectrophotometry, rheometry, and swelling kinetics, showed that the hydrogels are optically transparent, have good mechanical strength and a relatively low degree of swelling (<7%). In vitro doxycycline release from the hydrogel disks (0.25% w/v) was found to be biphasic with release half times of approximately 12 and 72h, respectively, with 80-100% released over a 7-day period. Permeation of doxycycline through vesicant wounded corneas was found to be 2.5 to 3.4 fold higher than non-wounded corneas. Histology and immunofluorescence studies showed a significant reduction of matrix metalloproteinase-9 (MMP-9) and improved healing of vesicant-exposed corneas by doxycycline hydrogels compared to a similar dose of doxycycline delivered in phosphate buffered saline (PBS, pH 7.4). In conclusion, the current studies demonstrate that the doxycycline-PEG hydrogels accelerate corneal wound healing after vesicant injury offering a therapeutic option for ocular mustard injuries.
Figures

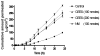

References
-
- Blanc PD. The legacy of war gas. Am J Med. 1999;106(6):689–690. - PubMed
-
- Saladi RN, Smith E, Persaud AN. Mustard: a potential agent of chemical warfare and terrorism. Clin Exp Dermatol. 2006;31(1):1–5. - PubMed
-
- Borak J, Sidell FR. Agents of chemical warfare: sulfur mustard. Ann Emerg Med. 1992;21(3):303–308. - PubMed
-
- Ghanei M, Harandi AA. Long term consequences from exposure to sulfur mustard: a review. Inhal Toxicol. 2007;19(5):451–456. - PubMed
-
- Papirmeister B. In: Medical Defense Against Mustard Gas: Toxic Mechanisms and Pharmacological Implications. Papirmeister B, Feister AJ, Robinson SI, Ford RD, editors. Boca Raton, Fl: CRC Press; 1991.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

