Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury
- PMID: 19853381
- PMCID: PMC2787843
- DOI: 10.1016/j.pain.2009.09.030
Peripheral and central sensitization in remote spinal cord regions contribute to central neuropathic pain after spinal cord injury
Abstract
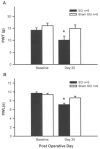
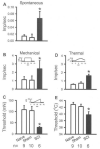
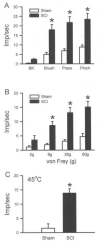
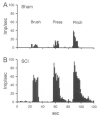
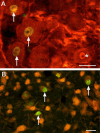
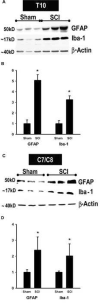
Central neuropathic pain (CNP) developing after spinal cord injury (SCI) is described by the region affected: above-level, at-level and below-level pain occurs in dermatomes rostral, at/near, or below the SCI level, respectively. People with SCI and rodent models of SCI develop above-level pain characterized by mechanical allodynia and thermal hyperalgesia. Mechanisms underlying this pain are unknown and the goals of this study were to elucidate components contributing to the generation of above-level CNP. Following a thoracic (T10) contusion, forelimb nociceptors had enhanced spontaneous activity and were sensitized to mechanical and thermal stimulation of the forepaws 35 days post-injury. Cervical dorsal horn neurons showed enhanced responses to non-noxious and noxious mechanical stimulation as well as thermal stimulation of receptive fields. Immunostaining dorsal root ganglion (DRG) cells and cord segments with activating transcription factor 3 (ATF3, a marker for neuronal injury) ruled out neuronal damage as a cause for above-level sensitization since few C8 DRG cells expressed AFT3 and cervical cord segments had few to no ATF3-labeled cells. Finally, activated microglia and astrocytes were present in thoracic and cervical cord at 35 days post-SCI, indicating a rostral spread of glial activation from the injury site. Based on these data, we conclude that peripheral and central sensitization as well as reactive glia in the uninjured cervical cord contribute to CNP. We hypothesize that reactive glia in the cervical cord release pro-inflammatory substances which drive chronic CNP. Thus a complex cascade of events spanning many cord segments underlies above-level CNP.
Figures


References
-
- Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;121:1–21. - PubMed
-
- Bennett AD, Everhart AW, Hulsebosch CE. Intrathecal administration of an NMDA or a non-NMDA receptor antagonist reduces mechanical but not thermal allodynia in a rodent model of chronic central pain after spinal cord injury. Brain Res. 2000;8591:72–82. - PubMed
-
- Bontioti E, Dahlin LB, Kataoka K, Kanje M. End-to-side nerve repair induces nuclear translocation of activating transcription factor 3. Scand J Plast Reconstr Surg Hand Surg. 2006;406:321–328. - PubMed
-
- Boyle DL, Moore J, Yang L, Sorkin LS, Firestein GS. Spinal adenosine receptor activation inhibits inflammation and joint destruction in rat adjuvant-induced arthritis. Arthritis Rheum. 2002;4611:3076–3082. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

