Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways
- PMID: 19853559
- PMCID: PMC3221601
- DOI: 10.1016/j.devcel.2009.09.011
Arrestin development: emerging roles for beta-arrestins in developmental signaling pathways
Abstract
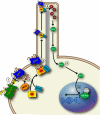
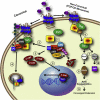
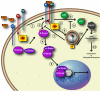
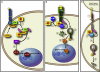
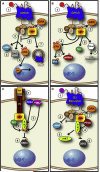
Arrestins were identified as mediators of G protein-coupled receptor (GPCR) desensitization and endocytosis. However, it is now clear that they scaffold many intracellular signaling networks to modulate the strength and duration of signaling by diverse types of receptors--including those relevant to the Hedgehog, Wnt, Notch, and TGFbeta pathways--and downstream kinases such as the MAPK and Akt/PI3K cascades. The involvement of arrestins in many discrete developmental signaling events suggests an indispensable role for these multifaceted molecular scaffolds.
Figures
References
-
- Ahn S, Shenoy SK, Wei H, Lefkowitz RJ. Differential kinetic and spatial patterns of beta-arrestin and G protein-mediated ERK activation by the angiotensin II receptor. J. Biol. Chem. 2004;279:35518–35525. - PubMed
-
- Barnes WG, Reiter E, Violin JD, Ren XR, Milligan G, Lefkowitz RJ. beta-Arrestin 1 and Galphaq/11 coordinately activate RhoA and stress fiber formation following receptor stimulation. J. Biol. Chem. 2005;280:8041–8050. - PubMed
-
- Beaulieu JM, Sotnikova TD, Marion S, Lefkowitz RJ, Gainetdinov RR, Caron MG. An Akt/beta-arrestin 2/PP2A signaling complex mediates dopaminergic neurotransmission and behavior. Cell. 2005;122:261–273. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

