LRF is an essential downstream target of GATA1 in erythroid development and regulates BIM-dependent apoptosis
- PMID: 19853566
- PMCID: PMC3134301
- DOI: 10.1016/j.devcel.2009.09.005
LRF is an essential downstream target of GATA1 in erythroid development and regulates BIM-dependent apoptosis
Abstract
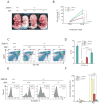
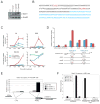
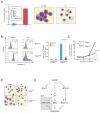
GATA-1-dependent transcription is essential for erythroid differentiation and maturation. Suppression of programmed cell death is also thought to be critical for this process; however, the link between these two features of erythropoiesis has remained elusive. Here, we show that the POZ-Krüppel family transcription factor, LRF (also known as Zbtb7a/Pokemon), is a direct target of GATA1 and plays an essential antiapoptotic role during terminal erythroid differentiation. We find that loss of Lrf leads to lethal anemia in embryos, due to increased apoptosis of late-stage erythroblasts. This programmed cell death is Arf and p53 independent and is instead mediated by upregulation of the proapoptotic factor Bim. We identify Lrf as a direct repressor of Bim transcription. In strong support of this mechanism, genetic Bim loss delays the lethality of Lrf-deficient embryos and rescues their anemia phenotype. Thus, our data define a key transcriptional cascade for effective erythropoiesis, whereby GATA-1 suppresses BIM-mediated apoptosis via LRF.
Figures



References
-
- Bouillet P, Zhang LC, Huang DC, Webb GC, Bottema CD, Shore P, Eyre HJ, Sutherland GR, Adams JM. Gene structure alternative splicing, and chromosomal localization of pro-apoptotic Bcl-2 relative Bim. Mamm Genome. 2001;12:163–168. - PubMed
-
- Bouillet P, Metcalf D, Huang DC, Tarlinton DM, Kay TW, Kontgen F, Adams JM, Strasser A. Proapoptotic Bcl-2 relative Bim required for certain apoptotic responses, leukocyte homeostasis, and to preclude autoimmunity. Science. 1999;286:1735–1738. - PubMed
-
- Cantor AB, Orkin SH. Transcriptional regulation of erythropoiesis: an affair involving multiple partners. Oncogene. 2002;21:3368–3376. - PubMed
-
- Costoya JA, Hobbs RM, Barna M, Cattoretti G, Manova K, Sukhwani M, Orwig KE, Wolgemuth DJ, Pandolfi PP. Essential role of Plzf in maintenance of spermatogonial stem cells. Nat Genet. 2004;36:653–659. - PubMed
-
- Crossley M, Tsang AP, Bieker JJ, Orkin SH. Regulation of the erythroid Kruppel-like factor (EKLF) gene promoter by the erythroid transcription factor GATA-1. J Biol Chem. 1994;269:15440–15444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

